SANCUSO Transdermal patch Ref.[8214] Active ingredients: Granisetron
Source: European Medicines Agency (EU) Revision Year: 2019 Publisher: Kyowa Kirin Holdings B.V., Bloemlaan 2, 2132NP Hoofddorp, The Netherlands, Tel: +31 (0) 237200822
Pharmacodynamic properties
Pharmacotherapeutic group: Antiemetics and antinauseants, serotonin (5HT3) antagonists
ATC code: A04AA02
Granisetron is a potent anti-emetic and highly selective antagonist of 5-hydroxytryptamine (5HT3 receptors). Pharmacological studies have demonstrated that granisetron is effective against nausea and vomiting as a result of cytostatic therapy. Radioligand binding studies have demonstrated that granisetron has negligible affinity for other receptor types, including 5HT1, 5HT2, 5HT4 and dopamine D2 binding sites.
A pivotal, randomised, double-blind, double-dummy, multinational Phase III study compared the efficacy, tolerability and safety of SANCUSO with that of 2 mg oral granisetron once daily in the prevention of nausea and vomiting in a total of 641 patients receiving multi-day chemotherapy. The study was designed to show non-inferiority of SANCUSO to oral granisetron.
The population randomised into the trial included 48% males and 52% females aged 16 to 86 years receiving moderately emetogenic (ME) or highly emetogenic (HE) multi-day chemotherapy. 78% of patients were white, with 12% Asian and 10% Hispanic/Latino.
The granisetron transdermal patch was applied 24 to 48 hours prior to the first dose of chemotherapy, and kept in place for 7 days. Oral granisetron was administered daily for the duration of the chemotherapy regimen, one hour prior to each dose of chemotherapy. Anti-emetic activity was assessed from the first administration until 24 hours after the start of the last day’s administration of the ME or HE chemotherapy regimen.
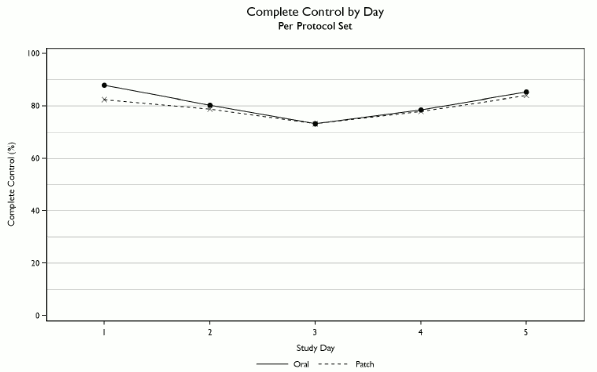
Non-inferiority of SANCUSO versus oral granisetron was confirmed, with complete control (CC) achieved in 60.2% of patients in the SANCUSO arm and 64.8% of patients receiving oral granisetron in the per protocol set (difference -4.89%; 95% confidence interval -12.91% to +3.13%; n=284 transdermal patch, n=298 oral). CC was defined as no vomiting and/or retching, no more than mild nausea and no rescue medicine from the first administration until 24 hours after the start of the last day’s administration of multi-day chemotherapy.
Due to the gradual increase in plasma levels of granisetron following application of the transdermal patch, initial plasma levels at the start of chemotherapy may be lower than 2 mg oral granisetron and a slower onset of efficacy may therefore be observed. Consequently, SANCUSO is indicated for use in patients where oral anti-emetic administration is complicated by factors making swallowing difficult.
Complete control by day is illustrated below.
In clinical trials with SANCUSO, there were no treatment-related effects on heart rate or blood pressure. Assessment of serial ECGs in patients showed no QT prolongation and no change in ECG morphology. The effect of SANCUSO on QTc interval was specifically evaluated in a blinded, randomised, parallel, placebo and positive (moxifloxacin) controlled thorough QTc trial with SANCUSO in 240 adult male and female subjects. No significant effect on QTc prolongation was observed for SANCUSO.
An assessment of transdermal patch adhesion in 621 patients receiving either active or placebo transdermal patches showed that less than 1% of transdermal patches became detached over the course of the 7 day period of transdermal patch application.
There is no clinical trial experience with SANCUSO and patients on chemotherapy for less than 3 consecutive days, or over multiple cycles of chemotherapy, or with high-dose chemotherapy prior to stem-cell transplantation.
Pharmacokinetic properties
Absorption
Granisetron crosses intact skin into the systemic circulation by a passive diffusion process. Following SANCUSO application, granisetron is absorbed slowly, with maximal concentrations reached between 24 and 48 hours.
Based on the measure of residual content of the transdermal patch after removal, approximately 65% of granisetron is delivered resulting in an average daily dose of 3.1 mg per day.
Concurrent administration of a single intravenous bolus of 0.01 mg/kg (maximum 1 mg) granisetron at the same time a SANCUSO transdermal patch was applied was investigated in healthy subjects. An initial peak in plasma concentrations of granisetron, attributable to the intravenous dose, was reached at 10 minutes post-administration. The known pharmacokinetic profile of the transdermal patch over the period of wear (7 days) was not affected.
Following consecutive application of two SANCUSO transdermal patches in healthy subjects, each for seven days, granisetron levels were maintained over the study period with evidence of minimal accumulation.
In a study designed to assess the effect of heat on the transdermal delivery of granisetron from SANCUSO in healthy subjects, a heat pad generating an average temperature of 42°C was applied over the transdermal patch for 4 hours each day over the 5 day period of wear. While application of the heat pad was associated with a minor and transient increase in the transdermal patch flux during the period of heat pad application, no overall increase in granisetron exposure was observed when compared to a control group.
In a pharmacokinetic study in healthy volunteers, where SANCUSO was applied for a period of 7 days, mean total exposure (AUC0-infinity) was 416 ng h/ml (range 55-1192 ng h/ml), with a between subject variability of 89%. Mean Cmax was 3.9 ng/ml (range 0.7-9.5 ng/ml), with a between subject variability of 77%. This variability is similar to the known high variability in granisetron pharmacokinetics after oral or intravenous administration.
Distribution
Granisetron is distributed with a mean volume of distribution of approximately 3 l/kg. Plasma protein binding is approximately 65%. Granisetron distributes freely between plasma and red blood cells.
Biotransformation
No differences in the metabolic profiles of granisetron were observed between the oral and transdermal uses.
Granisetron is mainly metabolised to 7-hydroxygranisetron and 9’N-desmethylgranisetron. In vitro studies using human liver microsomes indicate that CYP1A1 is the major enzyme responsible for the 7-hydroxylation of granisetron, whereas CYP3A4 contributes to 9’desmethylation.
Elimination
Granisetron is cleared primarily by hepatic metabolism. After intravenous dosing, the mean plasma clearance ranged from 33.4 to 75.7 l/h in healthy subjects and from 14.7 to 33.6 l/h in patients with wide inter-subject variability. The mean plasma half-life in healthy subjects is 4-6 hours and in patients is 9-12 hours. After transdermal patch application, the apparent granisetron plasma half-life in healthy subjects was prolonged to approximately 36 hours due to the slow absorption rate of granisetron through the skin.
In clinical studies conducted with SANCUSO, clearance in cancer patients was shown to be approximately half that of healthy subjects.
After intravenous injection, approximately 12% of the dose is excreted unchanged in the urine of healthy subjects in 48 hours. The remainder of the dose is excreted as metabolites, with 49% in the urine and 34% in the faeces.
Pharmacokinetics in special populations
The effects of gender on the pharmacokinetics of SANCUSO have not been specifically studied. No consistent gender effects on pharmacokinetics were observed in clinical studies with SANCUSO, with a large inter-individual variability reported in both sexes. Population PK modelling has confirmed the absence of a gender effect on the pharmacokinetics of SANCUSO.
Elderly
In a clinical study no differences were seen in the plasma pharmacokinetics of SANCUSO in male and female elderly subjects (≥65 years) compared with younger subjects (aged 18-45 years inclusive).
Renal or hepatic impairment
No clinical studies have been performed specifically to investigate the pharmacokinetics of SANCUSO in patients with renal or hepatic impairment. No clear relationship between renal function (as measured by creatinine clearance) and granisetron clearance was identified in population PK modelling. In patients with renal failure or hepatic impairment, the pharmacokinetics of granisetron were determined following a single 40 μg/kg intravenous dose of granisetron hydrochloride.
Hepatic impairment
In patients with hepatic impairment due to neoplastic liver involvement, total plasma clearance was approximately halved compared to patients without hepatic impairment. Given the wide variability in pharmacokinetic parameters of granisetron and the good tolerance well above the recommended dose, dose adjustment in patients with functional hepatic impairment is not necessary.
Renal impairment
No correlation between creatinine clearance and total clearance was observed in cancer patients, indicating no influence of renal impairment on the pharmacokinetics of granisetron.
Body Mass Index (BMI)
In a clinical study designed to assess granisetron exposure from SANCUSO in subjects with differing levels of body fat, using BMI as a surrogate measure for body fat, no differences were seen in the plasma pharmacokinetics of SANCUSO in male and female subjects with a low BMI [<19.5 kg/m² (males), <18.5 kg/m² (females)] and a high BMI (30.0 to 39.9 kg/m² inclusive) compared to a control group (BMI 20.0 to 24.9 kg/m2inclusive).
Paediatric population
No studies have been performed to investigate the pharmacokinetics of SANCUSO in paediatrics.
Preclinical safety data
Preclinical data did not reveal any special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, reproductive toxicity and genotoxicity. Carcinogenicity studies showed no special hazard for humans when used at the recommended dose. However, when administered in higher doses and over a prolonged period of time, the risk of carcinogenicity cannot be ruled out but with the short application period recommended for the transdermal delivery system, a carcinogenic risk for humans is not expected.
SANCUSO transdermal patches did not show any potential for photoirritation or photosensitivity when tested in vivo in guinea-pigs. Granisetron was not phototoxic when tested in vitro in a mouse fibroblast cell line. When tested for potential photogenotoxicity in vitro in a Chinese hamster ovary (CHO) cell line, granisetron increased the percentage of cells with chromosome damage following photoirradiation. Although, the clinical relevance of this finding is not completely clear, patients should be advised to cover the transdermal patch application site if there is a risk of exposure to sunlight throughout the period of wear and for 10 days following its removal (see section 4.4).
When tested for skin sensitising potential in guinea pigs, SANCUSO showed a low potential for irritancy.
A study in cloned human cardiac ion channels has shown that granisetron has the potential to affect cardiac repolarisation via blockade of hERG potassium channels. Granisetron has been shown to block both sodium and potassium channels, which could affect cardiac depolorisation and repolarisation and therefore PR, QRS, and QT intervals. These data help to clarify the mechanisms by which some of the ECG changes (particularly QT and QRS prolongation) associated with this class of substance can occur. However, no clinically relevant effects on ECG have been observed in clinical studies with SANCUSO, including a through QT study in 240 healthy subjects (section 5.1).
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.