SAYANA Suspension for injection Ref.[27683] Active ingredients: Medroxyprogesterone
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2020 Publisher: Pfizer Limited, Sandwich, Kent, CT13 9NJ, United Kingdom
4.3. Contraindications
- SAYANA PRESS is contra-indicated in patients with a known hypersensitivity to MPA or any of its excipients listed in section 6.1.
- SAYANA PRESS is contra-indicated if pregnancy is known or suspected.
- SAYANA PRESS is contra-indicated in women with known or suspected malignancy of the breast or genital organs.
- SAYANA PRESS is contra-indicated in patients with undiagnosed vaginal bleeding.
- SAYANA PRESS is contra-indicated in patients with severe hepatic impairment.
- SAYANA PRESS is contra-indicated in patients with metabolic bone disease.
- SAYANA PRESS is contra-indicated in patients with active thromboembolic disease and in patients with current or past history of cerebrovascular disease.
4.4. Special warnings and precautions for use
Warnings
Loss of Bone Mineral Density
Use of depot medroxyprogesterone acetate subcutaneous (DMPA-SC) reduces serum estrogen levels and is associated with significant loss of BMD due to the known effect of estrogen deficiency on the bone remodeling system. Bone loss is greater with increasing duration of use; however BMD appears to increase after DMPA-SC is discontinued and ovarian estrogen production increases.
This loss of BMD is of particular concern during adolescence and early adulthood, a critical period of bone accretion. It is unknown if use of DMPA-SC by younger women will reduce peak bone mass and increase the risk for fracture in later life i.e. after menopause.
A study to assess the BMD effects of DMPA-IM (Depo-Provera) in adolescent females showed that its use was associated with a statistically significant decline in BMD from baseline. After discontinuing DMPA-IM in adolescents, return of mean BMD to baseline values required 1.2 years at the lumbar spine, 4.6 years at the total hip and 4.6 years at the femoral neck (see section 5.1). However in some participants, BMD did not fully return to baseline during follow-up and the long-term outcome is not known in this group. In adolescents, SAYANA PRESS may be used, but only after other methods of contraception have been discussed with the patients and considered to be unsuitable or unacceptable.
A large observational study of predominantly adult female contraceptive users showed that use of DMPA- IM did not increase risk for bone fractures. Importantly, this study could not determine whether use of DMPA has an effect on fracture rate later in life (see section 5.1 – Relationship of fracture incidence to use of DMPA-IM by women of reproductive age).
In women of all ages, careful re-evaluation of the risks and benefits of treatment should be carried out in those who wish to continue use for more than 2 years. In particular, in women with significant lifestyle and/or medical risk factors for osteoporosis, other methods of contraception should be considered prior to use of SAYANA PRESS.
Significant risk factors for osteoporosis include:
- Alcohol abuse and/or tobacco use
- Chronic use of drugs that can reduce bone mass, e.g., anticonvulsants or corticosteroids
- Low body mass index or eating disorder, e.g., anorexia nervosa or bulimia
- Previous low trauma fracture
- Family history of osteoporosis
For further information on BMD changes in both adult and adolescent females, refer to section 5.1. Adequate intake of calcium and Vitamin D, whether from the diet or from supplements, is important for bone health in women of all ages.
Menstrual Irregularities
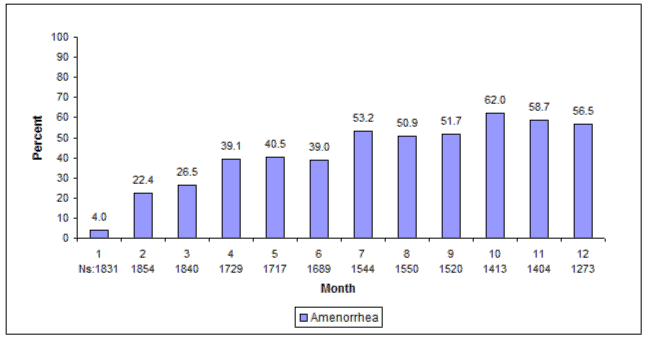
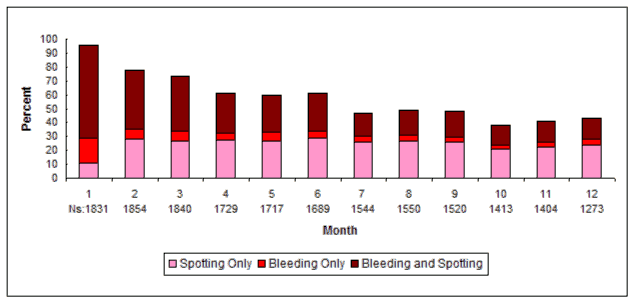
Most women using DMPA subcutaneous injection experienced alteration of menstrual bleeding patterns. Patients should be appropriately counseled concerning the likelihood of menstrual disturbance and the potential delay in return to ovulation. As women continued using DMPA subcutaneous injection, fewer experienced irregular bleeding and more experienced amenorrhea. After receiving the fourth dose, 39% of women experienced amenorrhea during month 6. During month twelve, 56.5% of women experienced amenorrhea. The changes in menstrual patterns from the three contraception trials are presented in Figures 1 and 2. Figure 1 shows the increase in the percentage of women experiencing amenorrhea over the 12 month study. Figure 2 presents the percentage of women experiencing spotting only, bleeding only, and bleeding and spotting over the same time period. In addition to amenorrhea, altered bleeding patterns included intermenstrual bleeding, menorrhagia and metrorrhagia. If abnormal bleeding associated with DMPA subcutaneous injection persists or is severe, appropriate investigation and treatment should be instituted.
Figure 1. Percent of DMPA subcutaneous injection -Treated Women with Amenorrhea per 30-Day Month Contraception Studies (ITT Population, N=2053):
Figure 2. Percent of DMPA subcutaneous injection -Treated Women with Bleeding and/or Spotting per 30-Day Month Contraception Studies (ITT Population, N=2053):
Cancer Risks
Long-term case-controlled surveillance of DMPA-IM 150 mg users found no overall increased risk of ovarian, liver, or cervical cancer and a prolonged, protective effect of reducing the risk of endometrial cancer in the population of users.
Breast cancer is rare among women under 40 years of age whether or not they use hormonal contraceptives.
Results from some epidemiological studies suggest a small difference in the risk of having the disease in current and recent users compared with never-users. Any excess risk in current and recent DMPA users is small in relation to the overall risk of breast cancer, particularly in young women (see below), and is not apparent after 10 years since last use. Duration of use does not seem to be important.
Possible number of additional cases of breast cancer diagnosed up to 10 years after stopping injectable progestogens*:
| Age at last use of DMPA | No of cases per 10,000 women who are never-users | Possible additional cases per 10,000 DMPA users |
|---|---|---|
| 20 | Less than 1 | Much less than 1 |
| 30 | 44 | 2-3 |
| 40 | 160 | 10 |
* based on use for 5 years"
Thromboembolic Disorders
Although MPA has not been causally associated with the induction of thrombotic or thromboembolic disorders, any patient who develops such an event, e.g. pulmonary embolism, cerebrovascular disease or retinal thrombosis or deep venous thrombosis, while undergoing therapy with SAYANA PRESS should not be readministered the drug. Women with a prior history of thromboembolic disorders have not been studied in clinical trials and no information is available that would support the safety of SAYANA PRESS use in this population.
Anaphylaxis and Anaphylactoid Reaction
If an anaphylactic reaction occurs appropriate therapy should be instituted. Serious anaphylactic reactions require emergency medical treatment.
Ocular Disorders
Medication should not be re-administered pending examination if there is a sudden partial or complete loss of vision or if there is a sudden onset of proptosis, diplopia, or migraine. If examination reveals papilledema or retinal vascular lesions, medication should not be re-administered.
Precautions
Weight Changes
Weight changes are common but unpredictable. In the phase 3 studies body weight was followed over 12 months. Half (50%) of women remained within 2.2 Kg of their initial body weight. 12% of women lost more than 2.2 Kg, and 38% of women gained more than 2.3 Kg.
Fluid Retention
There is evidence that progestogens may cause some degree of fluid retention, and as a result, caution should be exercised in treating any patient with a pre-existing medical condition that might be adversely affected by fluid retention.
Return of Ovulation
Following a single dose of DMPA subcutaneous injection, the cumulative rate of return to ovulation as measured by plasma progesterone was 97.4% (38/39 patients) by one year after administration. After the 14-week therapeutic window, the earliest return to ovulation was one week, and the median time to ovulation was 30 weeks. Women should be counseled that there is a potential for delay in return to ovulation following use of the method, regardless of the duration of use. It is recognised, however, that amenorrhoea and/or irregular menstruation upon discontinuation of hormonal contraception may be due to an underlying disorder associated with menstrual irregularity especially polycystic ovarian syndrome.
Psychiatric Disorders
Depressed mood and depression are well-known undesirable effects of hormonal contraceptive use (see section 4.8). Depression can be serious and is a well-known risk factor for suicidal behaviour and suicide. Women should be advised to contact their physician in case of mood changes and depressive symptoms, including shortly after initiating the treatment.
Protection against Sexually Transmitted Infections
Women should be counselled that SAYANA PRESS does not protect against sexually transmitted infections (STIs) including HIV infection (AIDS) but equally, DMPA is a sterile injection and, used as directed, will not expose them to STIs. Safer sex practices including correct and consistent use of condoms reduce the transmission of STIs through sexual contact, including HIV.
The benefits of contraceptive options and their risks must be evaluated individually for each woman.
Carbohydrate/Metabolism
Some patients receiving progestogens may exhibit a decrease in glucose tolerance. Diabetic patients should be carefully observed while receiving such therapy.
Liver Function
If jaundice develops in any woman receiving SAYANA PRESS, consideration should be given to not re-administer the medication. (see section 4.3).
Hypertension and Lipid disorders
Limited evidence suggests that there is a small increased risk of cardiovascular events among women with hypertension or with lipid disorders who used progestogen-only injectables. If hypertension occurs under SAYANA PRESS treatment and/or the increase in hypertension cannot adequately be controlled by antihypertensive medication, treatment with SAYANA PRESS should be stopped. Additional risk factors for arterial thrombotic disorders include: Hypertension, smoking, age, lipid disorders, migraine, obesity, positive family history, cardiac valve disorders, atrial fibrillation.
SAYANA PRESS should be used cautiously in patients with one or more of these risk factors.
Other conditions
The following conditions have been reported both during pregnancy and during sex steroid use, but an association with the use of progestagens has not been established: jaundice and/or pruritus related to cholestasis; gallstone formation; porphyria; systemic lupus erythematosus; hemolytic uraemic syndrome; Sydenham’s chorea; herpes gestationis; otosclerosis-related hearing loss.
If any of the conditions/risk factors mentioned is present, the benefits of SAYANA PRESS use should be weighed against the possible risks for each individual woman and discussed with the woman before she decides to start using it. In the event of aggravation, exacerbation or first appearance of any of these conditions or risk factors, the woman should contact her physician. The physician should then decide on whether SAYANA PRESS use should be discontinued.
Laboratory Tests
The pathologist should be advised of progestogen therapy when relevant specimens are submitted. The physician should be informed that certain endocrine and liver function tests, and blood components might be affected by progestogen therapy:
- Plasma/urinary steroids are decreased (e.g. progesterone, estradiol, pregnanediol, testosterone, cortisol)
- Plasma and urinary gonadotropin levels are decreased (e.g., LH, FSH).
- Sex-hormone-binding-globulin (SHBG) concentrations are decreased.
Excipients
As this product contains methylparahydroxbenzoate and propylparahydroxbenzoate, it may cause allergic reactions (possibly delayed), and exceptionally, bronchospasm. This medicinal product contains less than 1 mmol sodium (23 mg) per 104 mg/0.65 ml, i.e. essentially ‘sodium-free’.
4.5. Interaction with other medicinal products and other forms of interaction
No interaction studies have been performed with SAYANA PRESS.
Interactions with other medical treatments (including oral anticoagulants) have rarely been reported, but causality has not been determined. The possibility of interactions should be borne in mind in patients receiving concurrent treatment with other drugs.
MPA is metabolized in vitro primarily by hydroxylation via the CYP3A4. Specific drug-drug interaction studies evaluating the clinical effects with CYP3A4 inducers or inhibitors on MPA have not been conducted and therefore the clinical effects of CYP3A4 inducers or inhibitors are unknown.
4.6. Fertility, pregnancy and lactation
Fertility
SAYANA PRESS is indicated for the prevention of pregnancy.
Women may experience a delay in return to fertility (conception) following discontinuation of SAYANA PRESS (see section 4.4).
Pregnancy
SAYANA PRESS is contraindicated in women who are pregnant. Some reports suggest an association between intrauterine exposure to progestational drugs in the first trimester of pregnancy and genital abnormalities in male and female fetuses. If SAYANA PRESS is used during pregnancy, or if the patient becomes pregnant while using this drug, the patient should be warned of the potential hazard to the fetus.
One study found that infants from unintentional pregnancies that occurred 1 to 2 months after injection of DMPA-IM (150 mg) were at an increased risk of low birth weight; this, in turn, has been associated with an increased risk of neonatal death. However, the overall risk of this is very low because pregnancies while on DMPA-IM (150 mg) are uncommon.
Children exposed to MPA in utero and followed to adolescence showed no evidence of any adverse effects on their health including their physical, intellectual, sexual or social development.
Lactation
Low detectable amounts of drug have been identified in the milk of mothers receiving MPA. In nursing mothers treated with DMPA-IM (150 mg), milk composition, quality, and amount are not adversely affected. Neonates and infants exposed to MPA from breast milk have been studied for developmental and behavioural effects through puberty. No adverse effects have been noted. However, due to limitations of the data regarding the effects of MPA in breastfed infants less than six weeks old, SAYANA PRESS should be given no sooner than six weeks post partum when the infant’s enzyme system is more developed.
4.7. Effects on ability to drive and use machines
SAYANA PRESS has no influence on the ability to drive and use machines.
4.8. Undesirable effects
Events from clinical trials
The table below provides a listing of adverse drug reactions with frequency based on all-causality data from clinical studies that enrolled 2053 women who received DMPA-SC for contraception. The most frequently (>5%) reported adverse drug reactions were headache (8.9%), metrorrhagia (7.1%), weight increased (6.9%), amenorrhoea (6.3%) and injection site reactions (any type, 6.1%).
Adverse reactions are listed according to the following categories. These are as follows: Very Common (≥1/10), Common (≥1/100 to <1/10), Uncommon (≥1/1,000 to <1/100), Rare (≥1/10,000 to <1/1,000), Frequency not known (cannot be estimated from the available data).
Events from post-marketing surveillance
In addition, adverse events of medical significance obtained from post-marketing data with the use of injectable DMPA (IM or SC) are also included in the list below:
| System organ class | Very Common | Common | Uncommon | Rare | Not known |
|---|---|---|---|---|---|
| Neoplasms benign, malignant and unspecified (including cysts and polyps) | Breast cancer (see section 4.4) | ||||
| Immune system disorders | Drug hypersensitivity (see section 4.4) | Anaphylactic reaction, Anaphylactoid reaction, Angioedema (see section 4.4) | |||
| Metabolism and nutrition disorders | Fluid retention (see section 4.4), Increased appetite, Decreased appetite | ||||
| Psychiatric disorders | Depression, Insomnia, Anxiety, Affective disorder, Irritability, Libido decreased | Nervousness, Emotional disorder, Anorgasmia | |||
| Nervous system disorders | Dizziness, Headache | Migraine, Somnolence | Seizure | ||
| Ear and labyrinth disorders | Vertigo | ||||
| Cardiac disorders | Tachycardia | ||||
| Vascular disorders | Hypertension (see section 4.4), Varicose vein, Hot flush | Pulmonary embolism, Embolism and thrombosis, (see section 4.4), Thrombophlebitis | |||
| Gastrointestinal disorders | Abdominal pain, Nausea | Abdominal distension | |||
| Hepatobiliary disorders | Jaundice, Hepatic function abnormal (see section 4.4) | ||||
| Skin and subcutaneous tissue disorders | Acne | Alopecia, Hirsutism, Dermatitis, Ecchymosis, Chloasma, Rash, Pruritus, Urticaria | Lipodystrophy acquired | Skin striae | |
| Musculoskeletal and connective tissue disorders | Back pain, Pain in extremity | Arthralgia, Muscle spasms | Osteoporosis, Osteoporotic fractures | ||
| Reproductive system & breast disorders | Menometrorrhagia, Metrorrhagia, Menorrhagia (see section 4.4), Dysmenorrhoea, Amenorrhoea, Vaginitis, Breast pain | Ovarian cyst, Uterine haemorrhage (irregular, increase, decrease), Vaginal discharge, Dyspareunia, Galactorrhoea, Vulvovaginal dryness, Premenstrual syndrome, Breast tenderness, Breast enlargement | |||
| General disorders and administration site conditions | Fatigue, Injection site reaction, Injection site persistent atrophy/Indentation/dimpling, Injection site nodule/lump, Injection site pain/ tenderness | Pyrexia | Asthenia | ||
| Investigations | Weight increased (see section 4.4), Smear cervix abnormal | Bone density decreased (see section 4.4), Glucose tolerance decreased (see section 4.4), Hepatic enzyme abnormal | Weight decreased (see section 4.4) |
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or Apple App Store.
6.2. Incompatibilities
Not applicable.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.