SAYANA Suspension for injection Ref.[27683] Active ingredients: Medroxyprogesterone
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2020 Publisher: Pfizer Limited, Sandwich, Kent, CT13 9NJ, United Kingdom
4.1. Therapeutic indications
SAYANA PRESS is indicated for long-term female contraception. Each subcutaneous injection prevents ovulation and provides contraception for at least 13 weeks (+/- 1 week). However, it should be taken into consideration that the return to fertility (ovulation) may be delayed for up to one year (see section 4.4).
Since loss of bone mineral density (BMD) may occur in females of all ages who use SAYANA PRESS long-term (see section 4.4), a risk/benefit assessment, which also takes into consideration the decrease in BMD that occurs during pregnancy and/or lactation, should be considered.
Use in Adolescents (12-18 years)
In adolescents, use of SAYANA PRESS is only indicated when other contraceptive methods are considered unsuitable or unacceptable, due to unknown long-term effects of bone loss associated with SAYANA PRESS during the critical period of bone accretion (see section 4.4).
SAYANA PRESS has not been studied in women under the age of 18 years but data are available for intramuscular depot-medroxyprogesterone acetate (DMPA-IM) 150mg in this population.
4.2. Posology and method of administration
SAYANA PRESS may be administered by a healthcare professional (HCP) or when considered appropriate by the HCP, self-injected by the patient, with medical follow up as necessary in accordance with local clinical guidance.
Administration of SAYANA PRESS should be initiated under the supervision of a healthcare professional (HCP). After proper training in injection technique and schedule of administration, patients may self-inject with SAYANA PRESS if their HCP determines that it is appropriate and with medical follow-up as necessary.
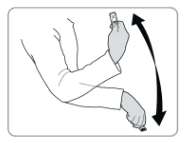
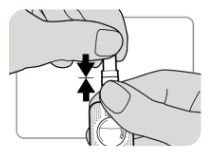
The SAYANA PRESS single-dose container should be at room temperature. It must be vigorously shaken just before use to ensure that the dose being given represents a uniform suspension. The contents are completely sealed inside the reservoir of the injector. The injector must be activated before use. The activation process pierces an internal seal so that the medicine can come out through the needle when the reservoir is squeezed. The liquid does not completely fill the reservoir. There is a small bubble of air above the liquid. The dose is administered as a subcutaneous injection (SC) into the anterior thigh or abdomen. When the injection is being given, the injector must be used with the needle downwards. This ensures that the full dose of liquid is delivered out through the needle. The medication should be injected slowly for 5-7 seconds.
Mixing the medicine:
- Ensure that the SAYANA PRESS single-dose container is at room temperature.
- Hold the injector firmly by the port.
- Shake the injector vigorously for at least 30 seconds to mix the medicine.
- The medicine should appear white and uniform. If it is not, discard the injector and use a new one.
- If you see liquid leaking out or any other problem, discard the injector and use a new one.
- If there is a delay before injecting, you must repeat the mixing step.
Activating the injector:
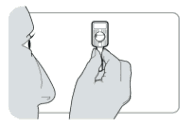
- Hold the injector firmly by the port, making sure the needle shield is pointing upwards. Take care not to squeeze the reservoir.
- Hold the needle shield with the other hand.
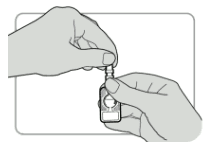
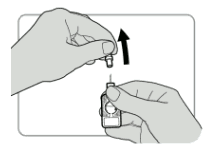
- Push the needle shield firmly towards the port until it will go no further. The injector is now activated.
- Pull the needle shield off and discard it.
Please refer to the Instructions for Use included with the Patient Leaflet for full details on preparing and giving an injection.
Adults
First Injection
To provide contraceptive cover in the first cycle of use, an injection of 104 mg SC should be given during the first five days of a normal menstrual cycle. If the injection is carried out according to these instructions, no additional contraceptive measure is required.
Further doses
The second and subsequent injections should be given at 13 week intervals, as long as the injection is given no later than seven days after this time, no additional contraceptive measures (e.g. barrier) are required. If the interval from the preceding injection is greater than 14 weeks (13 weeks plus 7 days) for any reason, then pregnancy should be excluded before the next injection is given. The efficacy of SAYANA PRESS depends on adherence to the recommended dosage schedule of administration.
Women should be re-evaluated periodically as clinically appropriate at least every year to determine if SAYANA PRESS is still the best option for them.
Post Partum
If the patient is not breast-feeding, the injection should be given within 5 days post partum (to increase assurance that the patient is not pregnant). If the injection is to be given at another time then the pregnancy should be excluded.
If the patient is breast-feeding, the injection should be given no sooner than six weeks post partum, when the infant’s enzyme system is more developed (see section 4.6).
There is evidence that women prescribed SAYANA PRESS in the immediate puerperium can experience prolonged and heavy bleeding. Because of this, the drug should be used with caution in the puerperium. Women who are considering use of the product immediately following delivery or termination should be advised that the risk of heavy or prolonged bleeding may be increased. Doctors are reminded that in the non breast-feeding, post partum patient, ovulation may occur as early as week 4.
Switching from other Methods of Contraception
When switching from other contraception methods, SAYANA PRESS should be given in a manner that ensures continuous contraceptive coverage based upon the mechanism of action of both methods, (e.g. patients switching from oral contraceptives should have their first injection of SAYANA PRESS within 7 days after their last active pill).
Hepatic impairment
The effect of hepatic disease on the pharmacokinetics of SAYANA PRESS is unknown. As SAYANA PRESS largely undergoes hepatic elimination it may be poorly metabolised in patients with severe hepatic insufficiency (see section 4.3).
Renal impairment
The effect of renal disease on the pharmacokinetics of SAYANA PRESS is unknown. No dosage adjustment should be necessary in women with renal insufficiency, since SAYANA PRESS is almost exclusively eliminated by hepatic metabolism.
Paediatric population
SAYANA PRESS is not indicated before menarche (see section 4.1). Data in adolescent females (12-18 years) is available for IM administration of MPA (see sections 4.4 and 5.1). Other than concerns about loss of BMD, the safety and effectiveness of SAYANA PRESS is expected to be the same for adolescents after menarche and adult females.
4.9. Overdose
No positive action is required other than cessation of therapy.
6.3. Shelf life
Unopened: 3 years.
Once opened: use immediately, discard any unused portion.
6.4. Special precautions for storage
Do not refrigerate or freeze.
6.5. Nature and contents of container
SAYANA PRESS suspension for injection is supplied in a single-dose container in the form of a pre-filled injector containing 0.65 ml. The injector comprises a linear low density polyethylene laminate reservoir with a siliconized AISI Type 304 Stainless Steel 23 gauge thin wall needle attached via a low density polyethylene port and valve.
The pack sizes are:
- one single-dose container
- 200 single-dose containers
Not all pack sizes may be marketed.
6.6. Special precautions for disposal and other handling
For single use only.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.