SENSIPAR Coated tablet Ref.[27431] Active ingredients: Cinacalcet
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
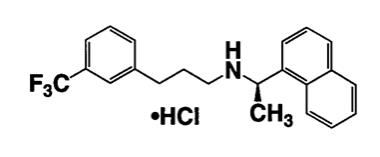
Sensipar tablets contain the hydrochloride salt of the active ingredient cinacalcet, a positive modulator of the calcium sensing receptor . The empirical formula for cinacalcet is C22H22F3N•HCl with a molecular weight of 393.9 g/mol (hydrochloride salt) and 357.4 g/mol (free base). It has one chiral center having an R-absolute configuration. The R-enantiomer is the more potent enantiomer and has been shown to be responsible for pharmacodynamic activity. The hydrochloride salt of cinacalcet is a white to off-white, crystalline solid that is soluble in methanol or 95% ethanol and slightly soluble in water.
The hydrochloride salt of cinacalcet is described chemically as N-[1-®()(1-naphthyl)ethyl]3[3-(trifluoromethyl)phenyl]-1-aminopropane hydrochloride and has the following structural formula:
Sensipar tablets are formulated as light-green, film-coated, oval-shaped tablets for oral administration in strengths of 30 mg, 60 mg, and 90 mg of cinacalcet as the free base equivalent (33 mg, 66 mg, and 99 mg as the hydrochloride salt, respectively).
Inactive Ingredients:
The following are the inactive ingredients in Sensipar tablets: pre-gelatinized starch, microcrystalline cellulose, povidone, crospovidone, colloidal silicon dioxide and magnesium stearate. Tablets are coated with color (Opadry II green), clear film coat (Opadry clear), and carnauba wax.
| Dosage Forms and Strengths |
|---|
|
Sensipar is available as film-coated tablets. Sensipar tablets are formulated as light-green, film-coated, oval-shaped tablets marked with “AMG” on one side and “30” or “60” or “90” on the opposite side of the 30 mg, 60 mg, or 90 mg strengths, respectively. |
| How Supplied |
|---|
|
Product: 50090-3529 NDC: 50090-3529-0 30 TABLET, COATED in a BOTTLE, PLASTIC Manufactured by: Amgen Inc., One Amgen Center Drive, Thousand Oaks, California 91320-1799 |
Drugs
| Drug | Countries | |
|---|---|---|
| SENSIPAR | Canada, New Zealand, United States, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.