SINGULAIR Tablet / Chewable Tablet / Granule Ref.[10692] Active ingredients: Montelukast
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
Montelukast sodium, the active ingredient in SINGULAIR, is a selective and orally active leukotriene receptor antagonist that inhibits the cysteinyl leukotriene CysLT1 receptor.
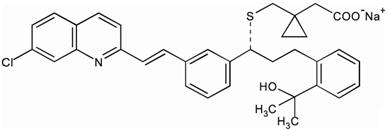
Montelukast sodium is described chemically as [R-(E)]-1-[[[1-[3-[2-(7-chloro-2-quinolinyl)ethenyl]phenyl]-3-[2-(1-hydroxy-1-methylethyl)phenyl]propyl]thio]methyl]cyclopropaneacetic acid, monosodium salt.
The empirical formula is C35H35ClNNaO3S, and its molecular weight is 608.18.
The structural formula is:
Montelukast sodium is a hygroscopic, optically active, white to off-white powder. Montelukast sodium is freely soluble in ethanol, methanol, and water and practically insoluble in acetonitrile.
Each 10-mg film-coated SINGULAIR tablet contains 10.4 mg montelukast sodium, which is equivalent to 10 mg of montelukast, and the following inactive ingredients: microcrystalline cellulose, lactose monohydrate, croscarmellose sodium, hydroxypropyl cellulose, and magnesium stearate. The film coating consists of: hydroxypropyl methylcellulose, hydroxypropyl cellulose, titanium dioxide, red ferric oxide, yellow ferric oxide, and carnauba wax.
Each 4-mg and 5-mg chewable SINGULAIR tablet contains 4.2 and 5.2 mg montelukast sodium, respectively, which are equivalent to 4 and 5 mg of montelukast, respectively. Both chewable tablets contain the following inactive ingredients: mannitol, microcrystalline cellulose, hydroxypropyl cellulose, red ferric oxide, croscarmellose sodium, cherry flavor, aspartame, and magnesium stearate.
Each packet of SINGULAIR 4-mg oral granules contains 4.2 mg montelukast sodium, which is equivalent to 4 mg of montelukast. The oral granule formulation contains the following inactive ingredients: mannitol, hydroxypropyl cellulose, and magnesium stearate.
| Dosage Forms and Strengths |
|---|
|
| How Supplied |
|---|
|
No. 3841 – SINGULAIR Oral Granules, 4 mg, are white granules with 500 mg net weight, packed in a child-resistant foil packet. They are supplied as follows: NDC 0006-3841-30 unit of use carton with 30 packets. No. 6628 – SINGULAIR Tablets, 4 mg, are pink, oval, bi-convex-shaped chewable tablets, with code MSD 711 on one side and SINGULAIR on the other. They are supplied as follows: NDC 0006-1711-31 unit of use high-density polyethylene (HDPE) bottles of 30 with a polypropylene child-resistant cap, an aluminum foil induction seal, and silica gel desiccant. No. 6543 – SINGULAIR Tablets, 5 mg, are pink, round, bi-convex-shaped chewable tablets, with code MSD 275 on one side and SINGULAIR on the other. They are supplied as follows: NDC 0006-9275-31 unit of use high-density polyethylene (HDPE) bottles of 30 with a polypropylene child-resistant cap, an aluminum foil induction seal, and silica gel desiccant. No. 6558 – SINGULAIR Tablets, 10 mg, are beige, rounded square-shaped, film-coated tablets, with code MSD 117 on one side and SINGULAIR on the other. They are supplied as follows: NDC 0006-9117-31 unit of use high-density polyethylene (HDPE) bottles of 30 with a polypropylene child-resistant cap, an aluminum foil induction seal, and silica gel desiccant NDC 0006-9117-54 unit of use high-density polyethylene (HDPE) bottles of 90 with a polypropylene child-resistant cap, an aluminum foil induction seal, and silica gel desiccant. Dist. by: Merck Sharp & Dohme Corp., a subsidiary of MERCK & CO., INC., Whitehouse Station, NJ 08889, USA |
Drugs
| Drug | Countries | |
|---|---|---|
| SINGULAIR | Austria, Brazil, Canada, Cyprus, Germany, Ecuador, Estonia, Spain, Finland, France, Hong Kong, Croatia, Ireland, Israel, Italy, Japan, Malta, Mexico, Netherlands, Poland, Romania, Singapore, Tunisia, Turkey, United Kingdom, United States, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.