SINGULAIR Tablet / Chewable Tablet / Granule Ref.[10692] Active ingredients: Montelukast
Source: FDA, National Drug Code (US) Revision Year: 2020
4. Contraindications
- Hypersensitivity to any component of this product.
5. Warnings and Precautions
5.1 Neuropsychiatric Events
Serious neuropsychiatric (NP) events have been reported with use of SINGULAIR. These postmarketing reports have been highly variable and included, but were not limited to, agitation, aggressive behavior or hostility, anxiousness, depression, disorientation, disturbance in attention, dream abnormalities, dysphemia (stuttering), hallucinations, insomnia, irritability, memory impairment, obsessive-compulsive symptoms, restlessness, somnambulism, suicidal thoughts and behavior (including suicide), tic, and tremor. NP events have been reported in adult, adolescent, and pediatric patients with and without a previous history of psychiatric disorder. NP events have been reported mostly during SINGULAIR treatment, but some were reported after SINGULAIR discontinuation. Animal studies showed that montelukast distributes into the brain in rats [see Clinical Pharmacology (12.3)]; however, the mechanisms underlying SINGULAIR-associated NP events are currently not well understood. Based upon the available data, it is difficult to identify risk factors for or quantify the risk of NP events with SINGULAIR use.
Because of the risk of NP events, the benefits of SINGULAIR may not outweigh the risks in some patients, particularly when the symptoms of disease may be mild and adequately treated with alternative therapies. Reserve use of SINGULAIR for patients with allergic rhinitis who have an inadequate response or intolerance to alternative therapies [see Indications and Usage (1.3)]. In patients with asthma or exercise-induced bronchoconstriction, consider the benefits and risks before prescribing SINGULAIR.
Discuss the benefits and risks of SINGULAIR use with patients and caregivers when prescribing SINGULAIR. Advise patients and/or caregivers to be alert for changes in behavior or for new NP symptoms when taking SINGULAIR. If changes in behavior are observed, or if new NP symptoms or suicidal thoughts and/or behavior occur, advise patients to discontinue SINGULAIR and contact a healthcare provider immediately. In many cases, symptoms resolved after stopping SINGULAIR therapy; however, in some cases symptoms persisted after discontinuation of SINGULAIR. Therefore, continue to monitor and provide supportive care until symptoms resolve. Re-evaluate the benefits and risks of restarting treatment with SINGULAIR if such events occur.
5.2 Acute Asthma
SINGULAIR is not indicated for use in the reversal of bronchospasm in acute asthma attacks, including status asthmaticus. Patients should be advised to have appropriate rescue medication available. Therapy with SINGULAIR can be continued during acute exacerbations of asthma. Patients who have exacerbations of asthma after exercise should have available for rescue a short-acting inhaled β-agonist.
5.3 Concomitant Corticosteroid Use
While the dose of inhaled corticosteroid may be reduced gradually under medical supervision, SINGULAIR should not be abruptly substituted for inhaled or oral corticosteroids.
5.4 Aspirin Sensitivity
Patients with known aspirin sensitivity should continue avoidance of aspirin or non-steroidal anti-inflammatory agents while taking SINGULAIR. Although SINGULAIR is effective in improving airway function in asthmatics with documented aspirin sensitivity, it has not been shown to truncate bronchoconstrictor response to aspirin and other non-steroidal anti-inflammatory drugs in aspirin-sensitive asthmatic patients [see Clinical Studies (14.1)].
5.5 Eosinophilic Conditions
Patients with asthma on therapy with SINGULAIR may present with systemic eosinophilia, sometimes presenting with clinical features of vasculitis consistent with Churg-Strauss syndrome, a condition which is often treated with systemic corticosteroid therapy. These events have been sometimes associated with the reduction of oral corticosteroid therapy. Physicians should be alert to eosinophilia, vasculitic rash, worsening pulmonary symptoms, cardiac complications, and/or neuropathy presenting in their patients. A causal association between SINGULAIR and these underlying conditions has not been established [see Adverse Reactions (6.2)].
5.6 Phenylketonuria
Phenylketonuric patients should be informed that the 4-mg and 5-mg chewable tablets contain phenylalanine (a component of aspartame), 0.674 and 0.842 mg per 4-mg and 5-mg chewable tablet, respectively.
6.1. Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice. In the following description of clinical trials experience, adverse reactions are listed regardless of causality assessment.
The most common adverse reactions (incidence ≥5% and greater than placebo; listed in descending order of frequency) in controlled clinical trials were: upper respiratory infection, fever, headache, pharyngitis, cough, abdominal pain, diarrhea, otitis media, influenza, rhinorrhea, sinusitis, otitis.
Adults and Adolescents 15 Years of Age and Older with Asthma
SINGULAIR has been evaluated for safety in approximately 2950 adult and adolescent patients 15 years of age and older in clinical trials. In placebo-controlled clinical trials, the following adverse experiences reported with SINGULAIR occurred in greater than or equal to 1% of patients and at an incidence greater than that in patients treated with placebo:
Table 1. Adverse Experiences Occurring in ≥1% of Patients with an Incidence Greater than that in Patients Treated with Placebo:
| SINGULAIR 10 mg/day (%) (n=1955) | Placebo (%) (n=1180) | |
|---|---|---|
| Body As A Whole | ||
| Pain, abdominal | 2.9 | 2.5 |
| Asthenia/fatigue | 1.8 | 1.2 |
| Fever | 1.5 | 0.9 |
| Trauma | 1.0 | 0.8 |
| Digestive System Disorders | ||
| Dyspepsia | 2.1 | 1.1 |
| Pain, dental | 1.7 | 1.0 |
| Gastroenteritis, infectious | 1.5 | 0.5 |
| Nervous System/Psychiatric | ||
| Headache | 18.4 | 18.1 |
| Dizziness | 1.9 | 1.4 |
| Respiratory System Disorders | ||
| Influenza | 4.2 | 3.9 |
| Cough | 2.7 | 2.4 |
| Congestion, nasal | 1.6 | 1.3 |
| Skin/Skin Appendages Disorder | ||
| Rash | 1.6 | 1.2 |
| Laboratory Adverse Experiences* | ||
| ALT increased | 2.1 | 2.0 |
| AST increased | 1.6 | 1.2 |
| Pyuria | 1.0 | 0.9 |
* Number of patients tested (SINGULAIR and placebo, respectively): ALT and AST, 1935, 1170; pyuria, 1924, 1159.
The frequency of less common adverse events was comparable between SINGULAIR and placebo.
The safety profile of SINGULAIR, when administered as a single dose for prevention of EIB in adult and adolescent patients 15 years of age and older, was consistent with the safety profile previously described for SINGULAIR.
Cumulatively, 569 patients were treated with SINGULAIR for at least 6 months, 480 for one year, and 49 for two years in clinical trials. With prolonged treatment, the adverse experience profile did not significantly change.
Pediatric Patients 6 to 14 Years of Age with Asthma
SINGULAIR has been evaluated for safety in 476 pediatric patients 6 to 14 years of age. Cumulatively, 289 pediatric patients were treated with SINGULAIR for at least 6 months, and 241 for one year or longer in clinical trials. The safety profile of SINGULAIR in the 8-week, double-blind, pediatric efficacy trial was generally similar to the adult safety profile. In pediatric patients 6 to 14 years of age receiving SINGULAIR, the following events occurred with a frequency ≥2% and more frequently than in pediatric patients who received placebo: pharyngitis, influenza, fever, sinusitis, nausea, diarrhea, dyspepsia, otitis, viral infection, and laryngitis. The frequency of less common adverse events was comparable between SINGULAIR and placebo. With prolonged treatment, the adverse experience profile did not significantly change.
The safety profile of SINGULAIR, when administered as a single dose for prevention of EIB in pediatric patients 6 years of age and older, was consistent with the safety profile previously described for SINGULAIR.
In studies evaluating growth rate, the safety profile in these pediatric patients was consistent with the safety profile previously described for SINGULAIR. In a 56-week, double-blind study evaluating growth rate in pediatric patients 6 to 8 years of age receiving SINGULAIR, the following events not previously observed with the use of SINGULAIR in this age group occurred with a frequency ≥2% and more frequently than in pediatric patients who received placebo: headache, rhinitis (infective), varicella, gastroenteritis, atopic dermatitis, acute bronchitis, tooth infection, skin infection, and myopia.
Pediatric Patients 2 to 5 Years of Age with Asthma
SINGULAIR has been evaluated for safety in 573 pediatric patients 2 to 5 years of age in single- and multiple-dose studies. Cumulatively, 426 pediatric patients 2 to 5 years of age were treated with SINGULAIR for at least 3 months, 230 for 6 months or longer, and 63 patients for one year or longer in clinical trials. In pediatric patients 2 to 5 years of age receiving SINGULAIR, the following events occurred with a frequency ≥2% and more frequently than in pediatric patients who received placebo: fever, cough, abdominal pain, diarrhea, headache, rhinorrhea, sinusitis, otitis, influenza, rash, ear pain, gastroenteritis, eczema, urticaria, varicella, pneumonia, dermatitis, and conjunctivitis.
Pediatric Patients 6 to 23 Months of Age with Asthma
Safety and effectiveness in pediatric patients younger than 12 months of age with asthma have not been established.
SINGULAIR has been evaluated for safety in 175 pediatric patients 6 to 23 months of age. The safety profile of SINGULAIR in a 6-week, double-blind, placebo-controlled clinical study was generally similar to the safety profile in adults and pediatric patients 2 to 14 years of age. In pediatric patients 6 to 23 months of age receiving SINGULAIR, the following events occurred with a frequency ≥2% and more frequently than in pediatric patients who received placebo: upper respiratory infection, wheezing; otitis media; pharyngitis, tonsillitis, cough; and rhinitis. The frequency of less common adverse events was comparable between SINGULAIR and placebo.
Adults and Adolescents 15 Years of Age and Older with Seasonal Allergic Rhinitis
SINGULAIR has been evaluated for safety in 2199 adult and adolescent patients 15 years of age and older in clinical trials. SINGULAIR administered once daily in the morning or in the evening had a safety profile similar to that of placebo. In placebo-controlled clinical trials, the following event was reported with SINGULAIR with a frequency ≥1% and at an incidence greater than placebo: upper respiratory infection, 1.9% of patients receiving SINGULAIR vs. 1.5% of patients receiving placebo. In a 4-week, placebo-controlled clinical study, the safety profile was consistent with that observed in 2-week studies. The incidence of somnolence was similar to that of placebo in all studies.
Pediatric Patients 2 to 14 Years of Age with Seasonal Allergic Rhinitis
SINGULAIR has been evaluated in 280 pediatric patients 2 to 14 years of age in a 2-week, multicenter, double-blind, placebo-controlled, parallel-group safety study. SINGULAIR administered once daily in the evening had a safety profile similar to that of placebo. In this study, the following events occurred with a frequency ≥2% and at an incidence greater than placebo: headache, otitis media, pharyngitis, and upper respiratory infection.
Adults and Adolescents 15 Years of Age and Older with Perennial Allergic Rhinitis
SINGULAIR has been evaluated for safety in 3357 adult and adolescent patients 15 years of age and older with perennial allergic rhinitis of whom 1632 received SINGULAIR in two, 6-week, clinical studies. SINGULAIR administered once daily had a safety profile consistent with that observed in patients with seasonal allergic rhinitis and similar to that of placebo. In these two studies, the following events were reported with SINGULAIR with a frequency ≥1% and at an incidence greater than placebo: sinusitis, upper respiratory infection, sinus headache, cough, epistaxis, and increased ALT. The incidence of somnolence was similar to that of placebo.
Pediatric Patients 6 Months to 14 Years of Age with Perennial Allergic Rhinitis
The safety in patients 2 to 14 years of age with perennial allergic rhinitis is supported by the safety in patients 2 to 14 years of age with seasonal allergic rhinitis. The safety in patients 6 to 23 months of age is supported by data from pharmacokinetic and safety and efficacy studies in asthma in this pediatric population and from adult pharmacokinetic studies.
6.2. Postmarketing Experience
The following adverse reactions have been identified during post-approval use of SINGULAIR. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and lymphatic system disorders: increased bleeding tendency, thrombocytopenia.
Immune system disorders: hypersensitivity reactions including anaphylaxis, hepatic eosinophilic infiltration.
Psychiatric disorders: including, but not limited to, agitation, aggressive behavior or hostility, anxiousness, depression, disorientation, disturbance in attention, dream abnormalities, dysphemia (stuttering), hallucinations, insomnia, irritability, memory impairment, obsessive-compulsive symptoms, restlessness, somnambulism, suicidal thinking and behavior (including suicide), tic, and tremor [see Boxed Warning, Warnings and Precautions (5.1)].
Nervous system disorders: drowsiness, paraesthesia/hypoesthesia, seizures.
Cardiac disorders: palpitations.
Respiratory, thoracic and mediastinal disorders: epistaxis, pulmonary eosinophilia.
Gastrointestinal disorders: diarrhea, dyspepsia, nausea, pancreatitis, vomiting.
Hepatobiliary disorders: Cases of cholestatic hepatitis, hepatocellular liver-injury, and mixed-pattern liver injury have been reported in patients treated with SINGULAIR. Most of these occurred in combination with other confounding factors, such as use of other medications, or when SINGULAIR was administered to patients who had underlying potential for liver disease such as alcohol use or other forms of hepatitis.
Skin and subcutaneous tissue disorders: angioedema, bruising, erythema multiforme, erythema nodosum, pruritus, Stevens-Johnson syndrome/toxic epidermal necrolysis, urticaria.
Musculoskeletal and connective tissue disorders: arthralgia, myalgia including muscle cramps.
Renal and urinary disorders: enuresis in children.
General disorders and administration site conditions: edema.
Patients with asthma on therapy with SINGULAIR may present with systemic eosinophilia, sometimes presenting with clinical features of vasculitis consistent with Churg-Strauss syndrome, a condition which is often treated with systemic corticosteroid therapy. These events have been sometimes associated with the reduction of oral corticosteroid therapy. Physicians should be alert to eosinophilia, vasculitic rash, worsening pulmonary symptoms, cardiac complications, and/or neuropathy presenting in their patients [see Warnings and Precautions (5.5)].
7. Drug Interactions
No dose adjustment is needed when SINGULAIR is co-administered with theophylline, prednisone, prednisolone, oral contraceptives, terfenadine, digoxin, warfarin, gemfibrozil, itraconazole, thyroid hormones, sedative hypnotics, non-steroidal anti-inflammatory agents, benzodiazepines, decongestants, and Cytochrome P450 (CYP) enzyme inducers [see Clinical Pharmacology (12.3)].
8.1. Pregnancy
Risk Summary
Available data from published prospective and retrospective cohort studies over decades with montelukast use in pregnant women have not established a drug-associated risk of major birth defects [see Data]. In animal reproduction studies, no adverse developmental effects were observed with oral administration of montelukast to pregnant rats and rabbits during organogenesis at doses approximately 100 and 110 times, respectively, the maximum recommended human daily oral dose (MRHDOD) based on AUCs [see Data].
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Poorly or moderately controlled asthma in pregnancy increases the maternal risk of perinatal adverse outcomes such as preeclampsia and infant prematurity, low birth weight, and small for gestational age.
Data
Human Data
Published data from prospective and retrospective cohort studies have not identified an association with SINGULAIR use during pregnancy and major birth defects. Available studies have methodologic limitations, including small sample size, in some cases retrospective data collection, and inconsistent comparator groups.
Animal Data
In embryo-fetal development studies, montelukast administered to pregnant rats and rabbits during organogenesis (gestation days 6 to 17 in rats and 6 to 18 in rabbits) did not cause any adverse developmental effects at maternal oral doses up to 400 and 300 mg/kg/day in rats and rabbits, respectively (approximately 100 and 110 times the AUC in humans at the MRHDOD, respectively).
8.2. Lactation
Risk Summary
A published clinical lactation study reports the presence of montelukast in human milk. Data available on the effects of the drug on infants, either directly [see Use in Specific Populations (8.4)] or through breast milk, do not suggest a significant risk of adverse events from exposure to SINGULAIR. The effects of the drug on milk production are unknown. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for SINGULAIR and any potential adverse effects on the breastfed infant from SINGULAIR or from the underlying maternal condition.
8.4. Pediatric Use
Safety and efficacy of SINGULAIR have been established in adequate and well-controlled studies in pediatric patients with asthma 6 to 14 years of age. Safety and efficacy profiles in this age group are similar to those seen in adults [see Adverse Reactions (6.1), Clinical Pharmacology, Special Populations (12.3), and Clinical Studies (14.1, 14.2)].
The efficacy of SINGULAIR for the treatment of seasonal allergic rhinitis in pediatric patients 2 to 14 years of age and for the treatment of perennial allergic rhinitis in pediatric patients 6 months to 14 years of age is supported by extrapolation from the demonstrated efficacy in patients 15 years of age and older with allergic rhinitis as well as the assumption that the disease course, pathophysiology and the drug’s effect are substantially similar among these populations.
The safety of SINGULAIR 4-mg chewable tablets in pediatric patients 2 to 5 years of age with asthma has been demonstrated by adequate and well-controlled data [see Adverse Reactions (6.1)]. Efficacy of SINGULAIR in this age group is extrapolated from the demonstrated efficacy in patients 6 years of age and older with asthma and is based on similar pharmacokinetic data, as well as the assumption that the disease course, pathophysiology and the drug’s effect are substantially similar among these populations. Efficacy in this age group is supported by exploratory efficacy assessments from a large, well-controlled safety study conducted in patients 2 to 5 years of age.
The safety of SINGULAIR 4-mg oral granules in pediatric patients 12 to 23 months of age with asthma has been demonstrated in an analysis of 172 pediatric patients, 124 of whom were treated with SINGULAIR, in a 6-week, double-blind, placebo-controlled study [see Adverse Reactions (6.1)]. Efficacy of SINGULAIR in this age group is extrapolated from the demonstrated efficacy in patients 6 years of age and older with asthma based on similar mean systemic exposure (AUC), and that the disease course, pathophysiology and the drug’s effect are substantially similar among these populations, supported by efficacy data from a safety trial in which efficacy was an exploratory assessment.
The safety of SINGULAIR 4-mg and 5-mg chewable tablets in pediatric patients aged 2 to 14 years with allergic rhinitis is supported by data from studies conducted in pediatric patients aged 2 to 14 years with asthma. A safety study in pediatric patients 2 to 14 years of age with seasonal allergic rhinitis demonstrated a similar safety profile [see Adverse Reactions (6.1)]. The safety of SINGULAIR 4-mg oral granules in pediatric patients as young as 6 months of age with perennial allergic rhinitis is supported by extrapolation from safety data obtained from studies conducted in pediatric patients 6 months to 23 months of age with asthma and from pharmacokinetic data comparing systemic exposures in patients 6 months to 23 months of age to systemic exposures in adults.
The safety and effectiveness in pediatric patients below the age of 12 months with asthma, 6 months with perennial allergic rhinitis, and 6 years with exercise-induced bronchoconstriction have not been established.
Growth Rate in Pediatric Patients
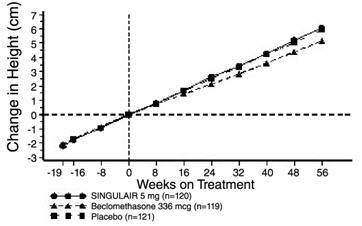
A 56-week, multi-center, double-blind, randomized, active- and placebo-controlled parallel group study was conducted to assess the effect of SINGULAIR on growth rate in 360 patients with mild asthma, aged 6 to 8 years. Treatment groups included SINGULAIR 5 mg once daily, placebo, and beclomethasone dipropionate administered as 168 mcg twice daily with a spacer device. For each subject, a growth rate was defined as the slope of a linear regression line fit to the height measurements over 56 weeks. The primary comparison was the difference in growth rates between SINGULAIR and placebo groups. Growth rates, expressed as least-squares (LS) mean (95% CI) in cm/year, for the SINGULAIR, placebo, and beclomethasone treatment groups were 5.67 (5.46, 5.88), 5.64 (5.42, 5.86), and 4.86 (4.64, 5.08), respectively. The differences in growth rates, expressed as least-squares (LS) mean (95% CI) in cm/year, for SINGULAIR minus placebo, beclomethasone minus placebo, and SINGULAIR minus beclomethasone treatment groups were 0.03 (-0.26, 0.31), -0.78 (-1.06, -0.49); and 0.81 (0.53, 1.09), respectively. Growth rate (expressed as mean change in height over time) for each treatment group is shown in FIGURE 1.
Figure 1. Change in Height (cm) from Randomization Visit by Scheduled Week (Treatment Group Mean ± Standard Error?footnote? of the Mean):
* The standard errors of the treatment group means in change in height are too small to be visible on the plot
8.5. Geriatric Use
Of the total number of subjects in clinical studies of montelukast, 3.5% were 65 years of age and over, and 0.4% were 75 years of age and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. The pharmacokinetic profile and the oral bioavailability of a single 10-mg oral dose of montelukast are similar in elderly and younger adults. The plasma half-life of montelukast is slightly longer in the elderly. No dosage adjustment in the elderly is required.
8.6. Renal Impairment
No dosage adjustment is recommended in patients with renal insufficiency [see Clinical Pharmacology (12.3)].
8.6. Hepatic Impairment
No dosage adjustment is required in patients with mild-to-moderate hepatic insufficiency [see Clinical Pharmacology (12.3)].
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.