SOOLANTRA Cream Ref.[8175] Active ingredients: Ivermectin
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2019 Publisher: Galderma (UK) Limited, Meridien House, 69-71 Clarendon Road, Watford, Herts., WD17 1DS, UK
Pharmacodynamic properties
Pharmacotherapeutic group: Other dermatological preparations, other dermatologicals
ATC code: D11AX22
Mechanism of action
Ivermectin is a member of the avermectin class. Avermectin has anti-inflammatory effects by inhibiting lipopolysaccharide-induced production of inflammatory cytokines. Anti-inflammatory properties of cutaneous ivermectin have been observed in animal models of skin inflammation. Ivermectin also causes death of parasites, primarily through binding selectively and with high affinity to glutamate-gated chloride channels, which occur in invertebrate nerve and muscle cells. The mechanism of action of Soolantra in treating the inflammatory lesions of rosacea is not known but may be linked to anti-inflammatory effects of ivermectin as well as causing the death of Demodex mites that have been reported to be a factor in inflammation of the skin.
Clinical efficacy and safety
Soolantra applied once daily at bedtime was evaluated in the treatment of inflammatory lesions of rosacea in two randomised, double-blind, vehicle-controlled clinical studies, which were identical in design. The studies were conducted in 1371 subjects aged 18 years and older who were treated once daily for 12 weeks with either Soolantra or vehicle.
Overall, 96% of subjects were Caucasian and 67% were female. Using the 5-point Investigator Global Assessment (IGA) scale, 79% of subjects were scored as moderate (IGA=3) and 21% scored as severe (IGA=4) at baseline.
The co-primary efficacy endpoints in both clinical studies were the success rate based on the IGA outcome (percentage of subjects "clear" and "almost clear" at Week 12 of the study) and absolute change from baseline in inflammatory lesion counts. The IGA scale is based on the following definitions:
Table 2. Investigator Global Assessment (IGA) scale:
| Grade | Score | Clinical Description |
|---|---|---|
| Clear | 0 | No inflammatory lesions present, no erythema |
| Almost Clear | 1 | Very few small papules/pustules, very mild erythema present |
| Mild | 2 | Few small papules/pustules, mild erythema |
| Moderate | 3 | Several small or large papules/pustules, moderate erythema |
| Severe | 4 | Numerous small and/or large papules/pustules, severe erythema |
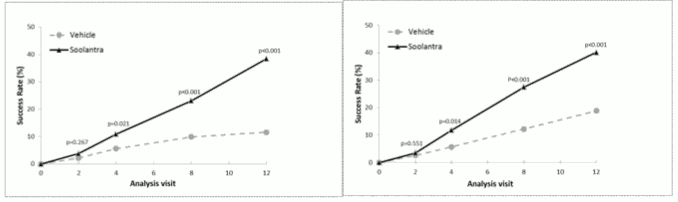
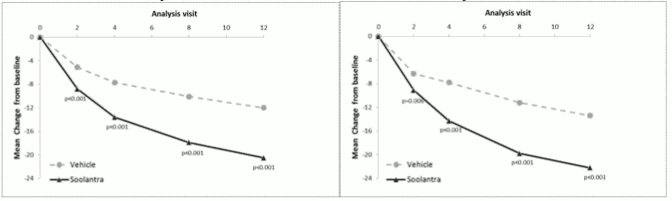
The results from both clinical studies demonstrated that Soolantra applied once daily for 12 weeks was statistically superior to vehicle cream in terms of IGA success rate and absolute change in inflammatory lesion counts (p<0.001, see table 3 and Figure 1, Figure 2, Figure 3 and Figure 4).
The following table and figures present efficacy outcomes from both studies.
Table 3. Efficacy Results:
| Study 1 | Study 2 | |||
|---|---|---|---|---|
| Soolantra (N=451) | Vehicle (N=232) | Soolantra (N=459) | Vehicle (N=229) | |
| Investigator Global Assessment | ||||
| Number (%) of Subjects Clear or Almost Clear in the IGA at Week 12 | 173 (38.4) | 27 (11.6) | 184 (40.1) | 43 (18.8) |
| Inflammatory Lesions | ||||
| Mean Inflammatory Lesion Count at Baseline | 31.0 | 30.5 | 33.3 | 32.2 |
| Mean Inflammatory Lesion Count at Week 12 | 10.6 | 18.5 | 11.0 | 18.8 |
| Mean Absolute Change (%Change) in Inflammatory Lesion Count from Baseline at Week 12 | -20.5 (-64.9) | -12.0 (-41.6) | -22.2 (-65.7) | -13.4 (-43.4) |
Figures 1 and 2. IGA Success Rates Over Time in weeks:
Figures 3 and 4. Mean Absolute Change in Inflammatory Lesion Counts from Baseline Over Time in weeks:
Soolantra was statistically superior to vehicle cream on the co-primary efficacy endpoints with a time to onset of efficacy of 4 weeks of treatment (p<0.05).
IGA was assessed during the 40-week extension of the two clinical studies and the percentages of subjects treated with Soolantra achieving an IGA score of 0 or 1 continued to increase up to Week 52. The Success Rate (IGA=0 or 1) at Week 52 was 71% and 76% in Studies 1 and 2, respectively.
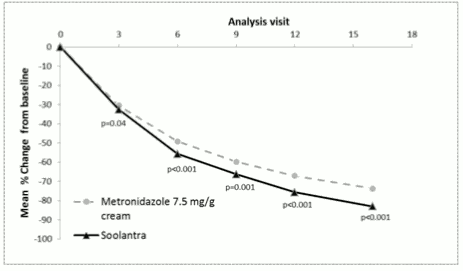
The efficacy and safety of the medicinal product in the treatment of inflammatory lesions of rosacea were also evaluated in a randomised, investigator-blinded, active-controlled clinical study. The study was conducted in 962 subjects aged 18 years and older who were treated for 16 weeks with either Soolantra once daily or Metronidazole 7.5 mg/g cream twice daily. In this study, 99.7% of subjects were Caucasian and 65.2% were female; on the IGA scale, 83.3% of subjects were scored as moderate (IGA=3) and 16.7% scored as severe (IGA=4) at baseline (see figure 5).
The results of the study demonstrated that Soolantra was statistically superior to Metronidazole 7.5 mg/g cream on the primary efficacy endpoint (Mean Percent Change in Inflammatory Lesion Counts) with a reduction of 83.0% and 73.7% from baseline after 16 weeks of treatment for the ivermectin and metronidazole groups respectively (p<0.001). The superiority of Soolantra at Week 16 was confirmed on Success Rate based on IGA and Absolute Change in Inflammatory Lesion Counts (secondary endpoints (p<0.001).
Figure 5. Mean percent change over time in weeks:
Approximately 300 subjects aged 65 years and older were treated over all clinical trials with the medicinal product. No meaningful differences in the efficacy and safety profile were observed between elderly subjects and subjects 18 to 65 years of age.
The safety profile, as described in section 4.8 remained stable over conditions of long-term use as observed in long-term treatments up to one year.
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with Soolantra in all subsets of the paediatric population in papulopustular rosacea (see section 4.2 for information on paediatric use).
Pharmacokinetic properties
Absorption
The absorption of ivermectin from Soolantra was evaluated in a clinical trial in adult subjects with severe papulopustular rosacea under maximal use conditions. At steady state (after 2 weeks of treatment), the highest mean (± standard deviation) plasma concentrations of ivermectin peaked within 10 ± 8 hours post-dose (Cmax: 2.1 ± 1.0 ng/mL range: 0.7-4.0 ng/mL) and the highest mean (± standard deviation) AUC0-24hr was 36± 16 ng.hr/mL (range: 14-75 ng.hr/mL). Ivermectin systemic exposure levels reached a plateau by two weeks of treatment (steady state conditions). In the longer treatment durations of the Phase 3 studies, ivermectin systemic exposure levels were similar to those observed after two weeks of treatment. At steady state conditions, the ivermectin systemic exposure levels (AUC0-24hr:36 ± 16 ng.hr/mL) were lower than those obtained following a single 6-mg oral dose of ivermectin in healthy volunteers (AUC0-24hr:134 ± 66 ng.hr/mL).
Distribution
An in vitro study demonstrated that ivermectin is greater than 99% bound to plasma proteins and is bound primarily to human serum albumin. No significant binding of ivermectin to erythrocytes was observed.
Biotransformation
In vitro studies using human hepatic microsomes and recombinant CYP450 enzymes have shown that ivermectin is primarily metabolized by CYP3A4.
In vitro studies show that ivermectin does not inhibit the CYP450 isoenzymes 1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 3A4, 4A11 or 2E1. Ivermectin does not induce CYP450 enzyme expression (1A2, 2B6, 2C9 or 3A4) in cultured human hepatocytes.
Two major metabolites of ivermectin were identified in a maximal use clinical pharmacokinetic study and assessed during Phase 2 clinical studies (3"-O-demethyl ivermectin and 4a-hydroxy ivermectin). Similar to the parent compound, metabolites reached steady state conditions by 2 weeks of treatment, with no evidence of accumulation up to 12 weeks. Furthermore, the metabolites systemic exposures (estimated with Cmax and AUC) obtained at steady state were much lower than those observed following oral administration of ivermectin.
Elimination
The terminal half-life averaged 6 days (mean: 145 hours, range 92-238 hours) in patients receiving a once daily cutaneous application of the medicinal product for 28 days, in the maximal use clinical pharmacokinetic study. Elimination is absorption-dependent following topical treatment with Soolantra. Pharmacokinetics of ivermectin have not been studied in patients with renal and hepatic impairment.
Preclinical safety data
Repeat-dose studies up to 9 months via dermal application of ivermectin 10 mg/g cream in minipigs have not shown toxic effects or local toxicity at systemic exposure levels comparable to clinical exposure.
Ivermectin is not genotoxic in a battery of in vitro and in vivo tests. A 2-year carcinogenicity study via dermal application of ivermectin 10 mg/g cream in mice did not show any increased tumour incidence.
Reproductive toxicity studies after oral administration of ivermectin showed teratogenic effects in rats (cleft palates) and rabbits (carpal flexures) at high doses (exposure margin to the NOAEL at least 70-fold compared to the clinical exposure).
The neonatal toxicity in oral rat studies was not related to in utero exposure but to postnatal exposure through maternal milk which resulted in high levels of ivermectin in the brain and in plasma of offspring.
Ivermectin 10 mg/g cream has evidence of being skin irritant, sensitizing and photosensitising in Guinea pigs, but is not phototoxic.
Environmental Risk Assessment (ERA)
Ivermectin is very toxic for invertebrates and a risk has been identified for the aquatic, sediment and the terrestrial compartment. Care should be taken in order to prevent environmental contamination, in particular in the aquatic media.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.