STENDRA Tablet Ref.[50965] Active ingredients: Avanafil
Source: FDA, National Drug Code (US) Revision Year: 2023
Product description
STENDRA (avanafil) is a selective inhibitor of cGMP-specific PDE5.
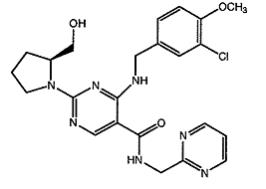
Avanafil is designated chemically as (S)-4-[(3-Chloro-4-methoxybenzyl)amino]-2-[2-(hydroxymethyl)-1-pyrrolidinyl]-N-(2-pyrimidinylmethyl)-5-pyrimidinecarboxamide and has the following structural formula:
Avanafil occurs as white crystalline powder, molecular formula C23H26ClN7O3 and molecular weight of 483.95 and is slightly soluble in ethanol, practically insoluble in water, soluble in 0.1 mol/L hydrochloric acid. STENDRA, for oral administration, is supplied as oval, pale yellow tablets containing 50 mg, 100 mg, or 200 mg avanafil debossed with dosage strengths. In addition to the active ingredient, avanafil, each tablet contains the following inactive ingredients: mannitol, fumaric acid, hydroxypropylcellulose, low substituted hydroxypropylcellulose, calcium carbonate, magnesium stearate, and ferric oxide yellow.
| Dosage Forms and Strengths |
|---|
|
STENDRA (avanafil) is supplied as oval, pale yellow tablets containing 50 mg, 100 mg, or 200 mg avanafil debossed with dosage strength. |
| How Supplied | ||||||||
|---|---|---|---|---|---|---|---|---|
|
STENDRA (avanafil) is supplied as oval, pale yellow tablets containing 50 mg, 100 mg, or 200 mg avanafil debossed with dosage strengths.
Manufactured for: Metuchen Pharmaceuticals, LLC, Freehold, NJ 07728 By: Sanofi Winthrope Industrie, Ambares, France |
Drugs
| Drug | Countries | |
|---|---|---|
| STENDRA | Turkey, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.