TIOGIVA Inhalation powder, hard capsule Ref.[28027] Active ingredients: Tiotropium
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2021 Publisher: Glenmark Pharmaceuticals Europe Limited, Laxmi House, 2B Draycott Avenue, Kenton, Middlesex, HA3 0BU, United Kingdom
4.1. Therapeutic indications
Tiotropium is indicated as a maintenance bronchodilator treatment to relieve symptoms of patients with chronic obstructive pulmonary disease (COPD).
4.2. Posology and method of administration
Posology
The recommended dosage of tiotropium bromide is inhalation of the contents of one capsule once daily with the dry powder inhaler the same time of day. To get a full daily dose, the patient must breath out completely. The patient should also inhale a second time from the same capsule.
The recommended dose should not be exceeded.
Tiogiva capsules are only for inhalation.
Tiogiva capsules must not be swallowed.
Tiotropium bromide should only be inhaled with the dry powder inhaler.
Special populations
Geriatric patients can use tiotropium bromide at the recommended dose.
Renally impaired patients can use tiotropium bromide at the recommended dose. For patients with moderate to severe impairment (creatinine clearance ≤ 50 ml/min) see section 4.4 and section 5.2.
Hepatically impaired patients can use tiotropium bromide at the recommended dose (see section 5.2).
Paediatric population
COPD
There is no relevant use in the paediatric population (below 18 years) in the indication stated under section 4.1.
Cystic fibrosis
The safety and efficacy of Tiogiva 18 microgram in children and adolescents has not been established. No data are available.
Method of administration/Instructions for handling and use
To ensure proper administration of the medicinal product the patient should be trained how to use the inhaler by the physician or by other healthcare professionals.
The dry powder inhaler is especially designed for Tiogiva capsules; patients must not use it to take any other medication.
The product comes with tiotropium capsules in blister packaging and depending on the pack also contains a “dry powder inhaler” (MRX003-R). The dry powder inhaler should be used with all accompanying capsules or, when used in combination with supplemental blister packs containing tiotropium capsules only, the dry powder inhaler could be used up to a maximum of 180 days.
The dry powder inhaler should only be used with the blister of capsules provided.
- Do not use your dry powder inhaler to take any other medicine.
- Do not use the dry powder inhaler for more than 180 days.
- Do not use the dry powder inhaler beyond the device expiry date.
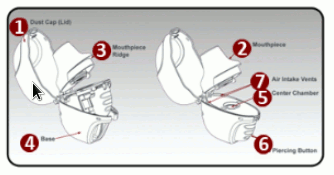
The parts of your dry powder inhaler include: (see Figure A)
- Dust cap (lid)
- Mouthpiece
- Mouthpiece ridge
- Base
- Centre chamber
- Piercing Button
- Air intake vents
Figure A:
Dispose of the dry powder inhaler once all accompanying capsules have been used or, when used in combination with supplemental blister packs containing tiotropium capsules, dispose of the dry powder inhaler after a period of 180 days.
Taking your full daily dose of medicine requires 4 main steps:
Step 1: Opening your dry powder inhaler
After removing your dry powder inhaler from the pouch:
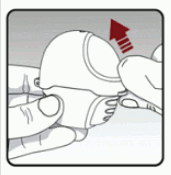
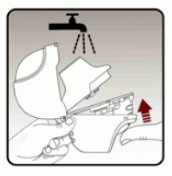
Open the dust cap (lid) by lifting the dust cap (lid). (see Figure B)
Figure B:
Pull the dust cap (lid) upwards away from the base to expose the mouthpiece. (see Figure C)
Figure C:
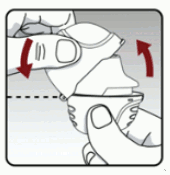
Open the mouthpiece by pulling the mouthpiece ridge up and away from the base so the centre chamber is showing. (see Figure D)
Figure D:
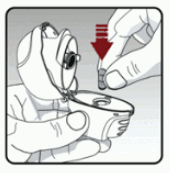
Step 2: Inserting the capsule into your dry powder inhaler
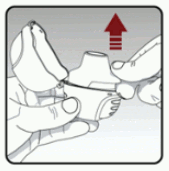
Remove a capsule from the blister (only immediately before use (see Blister Handling) and place the capsule in the centre chamber of your dry powder inhaler.
It does not matter which side of the capsule is up or down (See Figure E)
Figure E:
Close the mouthpiece firmly against the grey base until you hear a click. (see Figure F)
Leave the dust cap (lid) open.
Figure F:
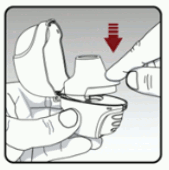
Step 3: Piercing the capsule:
- Hold your dry powder inhaler with the mouthpiece pointed up. (see Figure G)
- Press the piercing button once fully in until it stops, then release. This is how you make holes in the capsule so that you get your medicine when you breathe in.
- Do not press the piercing button more than one time.
- Do not push upward on the base while pressing the piercing button. This may cause the device to open, such as for cleaning (see Caring for and Storing Your Dry Powder Inhaler).
- Keep your inhaler in an upright position.
- Do not shake your dry powder inhaler.
- The piercing of the capsule may produce small capsule pieces. Some of these small pieces may pass through the screen of your MRX003-R into your mouth or throat when you breathe in your medicine. It is not harmful if these pieces are swallowed or inhaled.
Figure G:
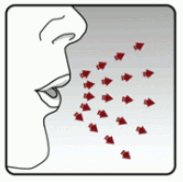
Step 4: Taking your full daily dose (2 inhalations from the same capsule)
Breathe out completely in 1 breath, emptying your lungs of any air. (see Figure H)
Important: Do not breathe into your dry powder inhaler.
Figure H:
With your next breath, take your medicine:
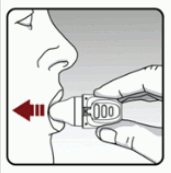
- Hold your head in an upright position while you are looking straight ahead. (see Figure I)
- Raise your dry powder inhaler to your mouth in a horizontal position. Do not block the air intake vents.
- Close your lips tightly around the mouthpiece.
- Breathe in slowly and deeply until your lungs are full. You should hear and/or feel the capsule vibrate (rattle).
- Hold your breath for a few seconds and, at the same time, take your dry powder inhaler out of your mouth.
- Breath normally again. Resume normal breathing. Repeat step 4 once more, in order to empty the capsule completely.
Figure I:
Important: Do not press the piercing button again.
Remember: To get your full medicine dose each day, you must breathe in 2 times from the same capsule. Make sure you breathe out completely each time before you breathe in from your dry powder inhaler.
Caring for and storing your Dry Powder Inhaler:
After taking your daily dose, open the mouthpiece and tip out the used capsule without touching it, into your rubbish bin.
- Remove any capsule pieces or powder build up, without touching it, by turning your dry powder inhaler upside-down and gently, but firmly, tapping it. (see Figure J)
- Close the mouthpiece and dust cap for storage.
Store the dry powder inhaler at ambient temperature (not more than 30°C).
Do not store your dry powder inhaler and tiotropium capsules (blisters) in a damp moist place. Always store tiotropium capsules in the sealed blisters.
Figure J:
Cleaning your dry powder inhaler:
Clean the dry powder inhaler monthly. (see Figure K).
- Do not use cleaning agents or detergents.
- Do not place your dry powder inhaler in the dishwasher for cleaning.
It takes 24 hours to air-dry your dry powder inhaler after you clean it:
- Do not use a hair dryer to dry your dry powder inhaler
- Do not use your dry powder inhaler when it is wet. If needed, you may clean the outside of the mouthpiece with a clean damp cloth.
Cleaning Steps:
- Open the dust cap and mouthpiece.
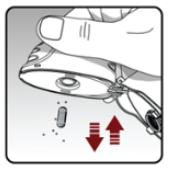
- Open the base by pushing the piercing button upwards.
- Look in the centre chamber for capsule pieces or powder build-up. If seen, tap out.
- Rinse your dry powder inhaler with warm water, pressing the button a few times so that the centre chamber and the piercing needle is under the running water. Check that any powder build-up or capsule pieces are removed.
- Dry your dry powder inhaler well by tipping the excess water out on a paper towel. Air-dry afterwards, leaving the dust cap, mouthpiece, and base open by fully spreading it out so that it dries completely. All parts of the dry powder inhaler should be completely dry prior to use.
Figure K:
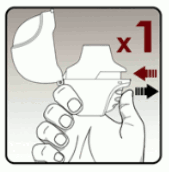
Blister handling:
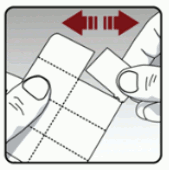
Each day, separate only 1 of the blisters from the blister card by tearing along the perforated line. (see Figure L)
Figure L:
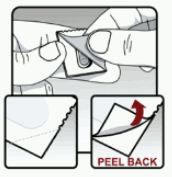
Remove the tiotropium capsule from the blister (see Figure M):
- Do not cut the foil or use sharp instruments to take out the capsule from the blister.
- Bend 1 of the blister corners with an arrow and separate the aluminium foil layers.
- Peel back the printed foil until you see the whole capsule. Do this only immediately before use.
- If you have opened more than 1 blister to the air, the extra capsule should not be used and should be thrown away.
Figure M:
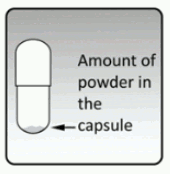
Each tiotropium capsule contains only a small amount of powder. (see Figure N) This is 1 full dose taken with two successive breaths.
Do not open the capsule or it may not work.
Figure N:
Additional Information:
- The dry powder inhaler is intended to be used by adults (minimum 18 years of age) who require pulmonary administration of a medicinal substance.
- There are no known contraindications for the dry powder inhaler.
- There are no special precautions for dry powder inhaler disposal.
- Do not use the device if it is visibly damaged or if an old capsule remains in the device.
- Consult a healthcare professional in case of any abnormal reactions to the dry powder inhaler use.
4.9. Overdose
High doses of tiotropium bromide may lead to anticholinergic signs and symptoms.
However, there were no systemic anticholinergic adverse effects following a single inhaled dose of up to 340 microgram tiotropium bromide in healthy volunteers. Additionally, no relevant adverse effects, beyond dry mouth, were observed following 7 day dosing of up to 170 microgram tiotropium bromide in healthy volunteers. In a multiple dose study in COPD patients with a maximum daily dose of 43 microgram tiotropium bromide over four weeks no significant undesirable effects have been observed.
Acute intoxication by inadvertent oral ingestion of tiotropium bromide capsules is unlikely due to low oral bioavailability.
6.3. Shelf life
24 months.
Use the capsule directly after opening of the blister pocket.
6.4. Special precautions for storage
Do not store above 30°C.
6.5. Nature and contents of container
Alu/Alu peel-off blister containing 10 capsules. The blisters are supplied in a cardboard box, depending on the pack the box will also contain a dry powder inhaler.
The dry powder inhaler is a single dose inhalation device made from acrylonitrile butadiene styrene (ABS) plastic materials and stainless steel.
Package sizes:
- Cardboard box containing 30 capsules (3 blisters) with a dry powder inhaler
- Cardboard box containing 60 capsules (6 blisters) with a dry powder inhaler
- Cardboard box containing 90 capsules (9 blisters) with a dry powder inhaler
- Cardboard box containing 30 capsules (3 blisters)
- Cardboard box containing 60 capsules (6 blisters)
- Cardboard box containing 90 capsules (9 blisters)
Not all pack sizes may be marketed.
6.6. Special precautions for disposal and other handling
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.