TOBREX 0.3% Ophthalmic solution Ref.[50522] Active ingredients: Tobramycin
Source: FDA, National Drug Code (US) Revision Year: 2022
Product description
TOBREX (tobramycin ophthalmic solution) 0.3% is a sterile topical ophthalmic antibiotic formulation prepared specifically for topical therapy of external ophthalmic infections.
Each mL of TOBREX (tobramycin ophthalmic solution) 0.3% contains:
Active: tobramycin 0.3% (3 mg).
Preservative: benzalkonium chloride 0.01% (0.1 mg).
Inactives: boric acid, purified water, sodium chloride, sodium hydroxide and/or sulfuric acid (to adjust pH), sodium sulfate, and tyloxapol. TOBREX (tobramycin ophthalmic solution) 0.3% has a pH range between 7.0 and 8.0 and an osmolality of 260-320 mOsm/kg.
Tobramycin is a water-soluble aminoglycoside antibiotic active against a wide variety of gram-negative and gram-positive ophthalmic pathogens.
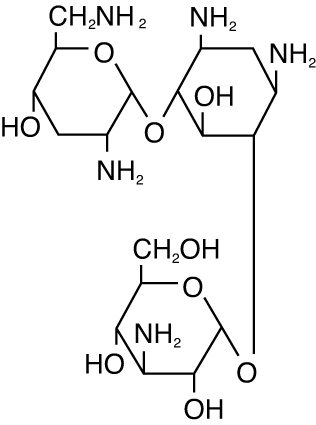
The chemical structure of tobramycin is:
Molecular Weight = 467.52
Molecular Formula: C18H37N5O9
Chemical Name: 0-{3-amino-3-deoxy-α-D-gluco-pyranosyl-(1→4)}-0-{2,6-diamino-2,3,6-trideoxy-α-D-ribohexo-pyranosyl-(1→6)}-2-deoxystreptamine.
| How Supplied |
|---|
|
TOBREX (tobramycin ophthalmic solution) 0.3% is supplied as a 5 mL sterile solution, packaged in a 8 mL low density polyethylene white bottle and natural dispensing plug and white polypropylene closure as follows: 5 mL containing tobramycin 0.3% (3 mg/mL) NDC 0065-0643-05 Distributed by: Novartis Pharmaceuticals Corporation, East Hanover, New Jersey 07936 |
Drugs
| Drug | Countries | |
|---|---|---|
| TOBREX | Austria, Australia, Brazil, Canada, Cyprus, Ecuador, Estonia, Spain, Finland, France, Hong Kong, Croatia, Israel, Lithuania, Malta, Mexico, Nigeria, Netherlands, New Zealand, Poland, Romania, Singapore, Tunisia, Turkey, United States, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.