TRIUMEQ Film-coated tablet Ref.[107695] Active ingredients: Abacavir Dolutegravir Lamivudine Lamivudine, Abacavir and Dolutegravir
Source: European Medicines Agency (EU) Revision Year: 2023 Publisher: ViiV Healthcare BV, Van Asch van Wijckstraat 55H, 3811 LP Amersfoort, Netherlands
4.3. Contraindications
Hypersensitivity to the active substances or to any of the excipients listed in section 6.1.
Co-administration with medicinal products with narrow therapeutic windows, that are substrates of organic cation transporter (OCT) 2, including but not limited to fampridine (also known as dalfampridine; see section 4.5).
4.4. Special warnings and precautions for use
Hypersensitivity reactions (see section 4.8)
Both abacavir and dolutegravir are associated with a risk for hypersensitivity reactions (HSR) (see section 4.8), and share some common features such as fever and/or rash with other symptoms indicating multi-organ involvement. Clinically it is not possible to determine whether a HSR with Triumeq would be caused by abacavir or dolutegravir. Hypersensitivity reactions have been observed more commonly with abacavir, some of which have been life-threatening, and in rare cases fatal, when not managed appropriately. The risk for abacavir HSR to occur is high for patients who test positive for the HLA-B*5701 allele. However, abacavir HSRs have been reported at a low frequency in patients who do not carry this allele.
Therefore, the following should always be adhered to:
- HLA-B*5701 status must always be documented prior to initiating therapy.
- Triumeq should never be initiated in patients with a positive HLA-B*5701 status, nor in patients with a negative HLA-B*5701 status who had a suspected abacavir HSR on a previous abacavircontaining regimen.
- Triumeq must be stopped without delay, even in the absence of the HLA-B*5701 allele, if an HSR is suspected. Delay in stopping treatment with Triumeq after the onset of hypersensitivity may result in an immediate and life-threatening reaction. Clinical status including liver aminotransferases and bilirubin should be monitored.
- After stopping treatment with Triumeq for reasons of a suspected HSR, Triumeq or any other medicinal product containing abacavir or dolutegravir must never be re-initiated.
- Restarting abacavir containing products following a suspected abacavir HSR can result in a prompt return of symptoms within hours. This recurrence is usually more severe than on initial presentation, and may include life-threatening hypotension and death.
- In order to avoid restarting abacavir and dolutegravir, patients who have experienced a suspected HSR should be instructed to dispose of their remaining Triumeq tablets.
Clinical description of HSRs
Hypersensitivity reactions have been reported in <1% of patients treated with dolutegravir in clinical studies, and were characterized by rash, constitutional findings, and sometimes, organ dysfunction, including severe liver reactions.
Abacavir HSR has been well characterised through clinical studies and during post marketing follow-up. Symptoms usually appeared within the first six weeks (median time to onset 11 days) of initiation of treatment with abacavir, although these reactions may occur at any time during therapy.
Almost all HSR to abacavir will include fever and/or rash. Other signs and symptoms that have been observed as part of abacavir HSR are described in detail in section 4.8 (Description of selected adverse reactions), including respiratory and gastrointestinal symptoms. Importantly, such symptoms may lead to misdiagnosis of HSR as respiratory disease (pneumonia, bronchitis, pharyngitis), or gastroenteritis. The symptoms related to this HSR worsen with continued therapy and can be life- threatening. These symptoms usually resolve upon discontinuation of abacavir.
Rarely, patients who have stopped abacavir for reasons other than symptoms of HSR have also experienced life-threatening reactions within hours of re-initiating abacavir therapy (see Section 4.8 Description of selected adverse reactions). Restarting abacavir in such patients must be done in a setting where medical assistance is readily available.
Weight and metabolic parameters
An increase in weight and in levels of blood lipids and glucose may occur during antiretroviral therapy. Such changes may in part be linked to disease control and lifestyle. For lipids and weight, there is in some cases evidence for a treatment effect. For monitoring of blood lipids and glucose reference is made to established HIV treatment guidelines. Lipid disorders should be managed as clinically appropriate.
Liver disease
The safety and efficacy of Triumeq has not been established in patients with significant underlying liver disorders. Triumeq is not recommended in patients with moderate to severe hepatic impairment (see sections 4.2 and 5.2).
Patients with pre-existing liver dysfunction, including chronic active hepatitis have an increased frequency of liver function abnormalities during combination antiretroviral therapy, and should be monitored according to standard practice. If there is evidence of worsening liver disease in such patients, interruption or discontinuation of treatment must be considered.
Patients with chronic hepatitis B or C
Patients with chronic hepatitis B or C and treated with combination antiretroviral therapy are at an increased risk of severe and potentially fatal hepatic adverse reactions. In case of concomitant antiviral therapy for hepatitis B or C, please refer also to the relevant product information for these medicinal products.
Triumeq includes lamivudine, which is active against hepatitis B. Abacavir and dolutegravir lack such activity. Lamivudine monotherapy is generally not considered an adequate treatment for hepatitis B, since the risk for hepatitis B resistance development is high. If Triumeq is used in patients co-infected with hepatitis B an additional antiviral is, therefore, generally needed. Reference should be made to treatment guidelines.
If Triumeq is discontinued in patients co-infected with hepatitis B virus, periodic monitoring of both liver function tests and markers of HBV replication is recommended, as withdrawal of lamivudine may result in an acute exacerbation of hepatitis.
Immune Reactivation Syndrome
In HIV-infected patients with severe immune deficiency at the time of institution of combination antiretroviral therapy (CART), an inflammatory reaction to asymptomatic or residual opportunistic pathogens may arise and cause serious clinical conditions, or aggravation of symptoms. Typically, such reactions have been observed within the first few weeks or months of initiation of CART. Relevant examples are cytomegalovirus retinitis, generalised and/or focal mycobacterial infections, and Pneumocystis jirovecii pneumonia (often referred to as PCP). Any inflammatory symptoms should be evaluated and treatment instituted when necessary. Autoimmune disorders (such as Graves' disease and autoimmune hepatitis) have also been reported to occur in the setting of immune reactivation; however, the reported time to onset is more variable and these events can occur many months after initiation of treatment.
Liver chemistry elevations consistent with immune reconstitution syndrome were observed in some hepatitis B and/or C co-infected patients at the start of dolutegravir therapy. Monitoring of liver chemistries is recommended in patients with hepatitis B and/or C co-infection. (See ‘Patients with chronic hepatitis B or C’ earlier in this section and also see section 4.8).
Mitochondrial dysfunction following exposure in utero
Nucleoside and nucleotide analogues may impact mitochondrial function to a variable degree, which is most pronounced with stavudine, didanosine and zidovudine. There have been reports of mitochondrial dysfunction in HIV-negative infants exposed in utero and/or post-natally to nucleoside analogues, these have predominantly concerned treatment with regimens containing zidovudine. The main adverse reactions reported are haematological disorders (anaemia, neutropenia), and metabolic disorders (hyperlactatemia, hyperlipasemia). These reactions have often been transitory. Some lateonset neurological disorders have been reported rarely (hypertonia, convulsion, abnormal behaviour). Whether such neurological disorders are transient or permanent is currently unknown. These findings should be considered for any child exposed in utero to nucleoside and nucleotide analogues, who presents with severe clinical findings of unknown aetiology, particularly neurologic findings. These findings do not affect current national recommendations to use antiretroviral therapy in pregnant women to prevent vertical transmission of HIV.
Cardiovascular events
Although the available data from clinical and observational studies with abacavir show inconsistent results, several studies suggest an increased risk of cardiovascular events (notably myocardial infarction) in patients treated with abacavir. Therefore, when prescribing Triumeq, action should be taken to minimise all modifiable risk factors (e.g. smoking, hypertension, and hyperlipidaemia). In addition, alternative treatment options to the abacavir containing regimen should be considered when treating patients with a high cardiovascular risk.
Osteonecrosis
Although the aetiology is considered to be multifactorial (including corticosteroid use, bisphosphonates, alcohol consumption, severe immunosuppression, higher body mass index), cases of osteonecrosis have been reported particularly in patients with advanced HIV-disease and/or long-term exposure to CART. Patients should be advised to seek medical advice if they experience joint aches and pain, joint stiffness or difficulty in movement.
Opportunistic infections
Patients should be advised that Triumeq or any other antiretroviral therapy does not cure HIV infection and that they may still develop opportunistic infections and other complications of HIV infection. Therefore, patients should remain under close clinical observation by physicians experienced in the treatment of these associated HIV diseases.
Administration in subjects with moderate renal impairment
Patients with a creatinine clearance between 30 and 49 mL/min receiving Triumeq may experience a 1.6-to 3.3-fold higher lamivudine exposure (AUC) than patients with a creatinine clearance ≥50 mL/min. There are no safety data from randomised, controlled trials comparing Triumeq to the individual components in patients with a creatinine clearance between 30 and 49 mL/min who received dose-adjusted lamivudine. In the original lamivudine registrational trials in combination with zidovudine, higher lamivudine exposures were associated with higher rates of haematologic toxicities (neutropenia and anaemia), although discontinuations due to neutropenia or anaemia each occurred in <1% of subjects. Other lamivudine-related adverse events (such as gastro-intestinal and hepatic disorders) may occur.
Patients with a sustained creatinine clearance between 30 and 49 mL/min who receive Triumeq should be monitored for lamivudine-related adverse events, notably hematologic toxicities. If new or worsening neutropenia or anaemia develop, a dose adjustment of lamivudine, per lamivudine prescribing information, is indicated, which cannot be achieved with Triumeq. Triumeq should be discontinued and the individual components should be used to construct the treatment regimen.
Drug resistance
The use of Triumeq is not recommended for patients with integrase inhibitor resistance. This is because the recommended dose of dolutegravir is 50 mg twice daily for adult patients with resistance to integrase inhibitors and there are insufficient data to recommend a dose of dolutegravir in integrase inhibitor resistant adolescents, children and infants.
Drug interactions
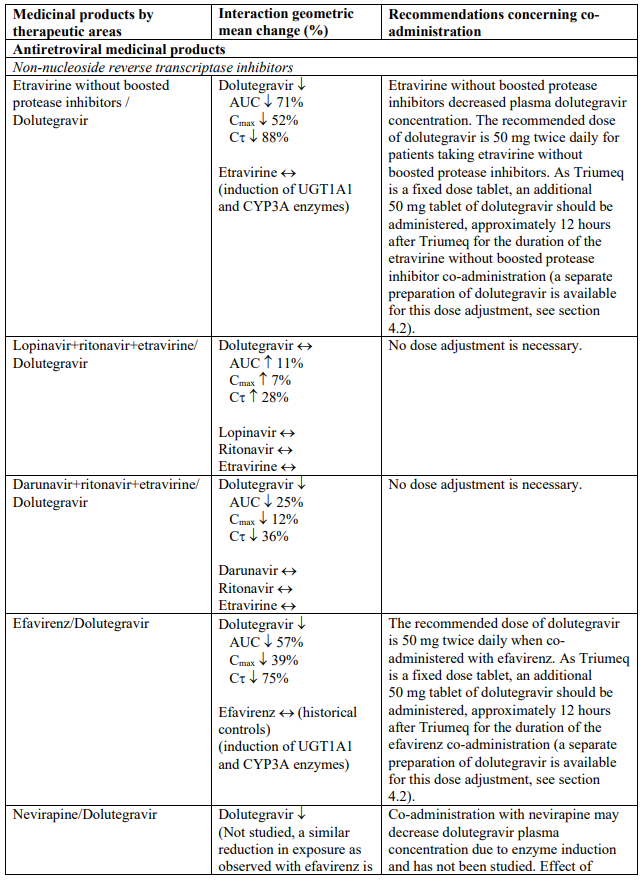
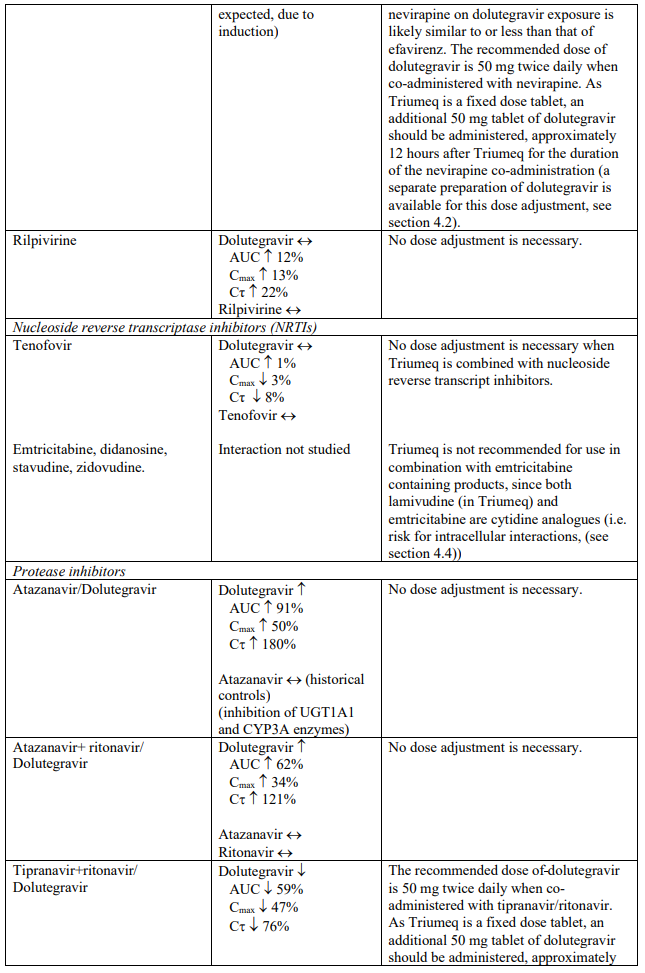
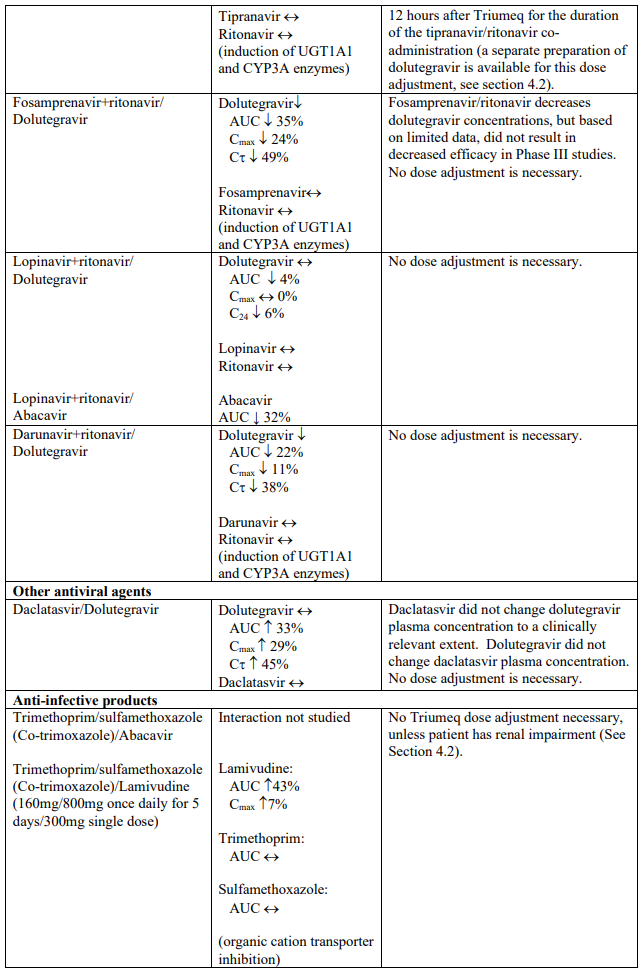
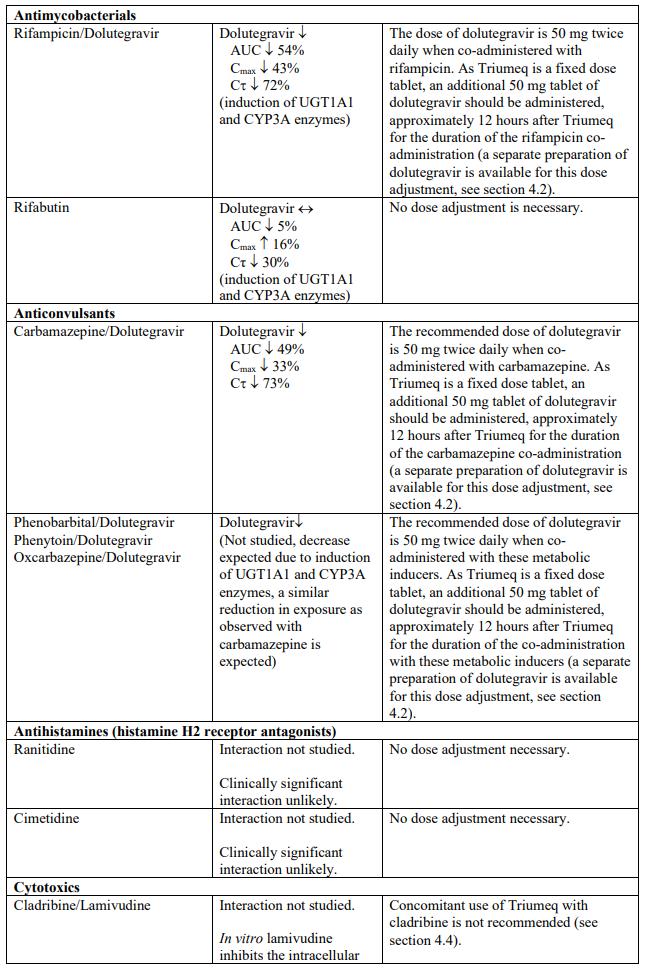
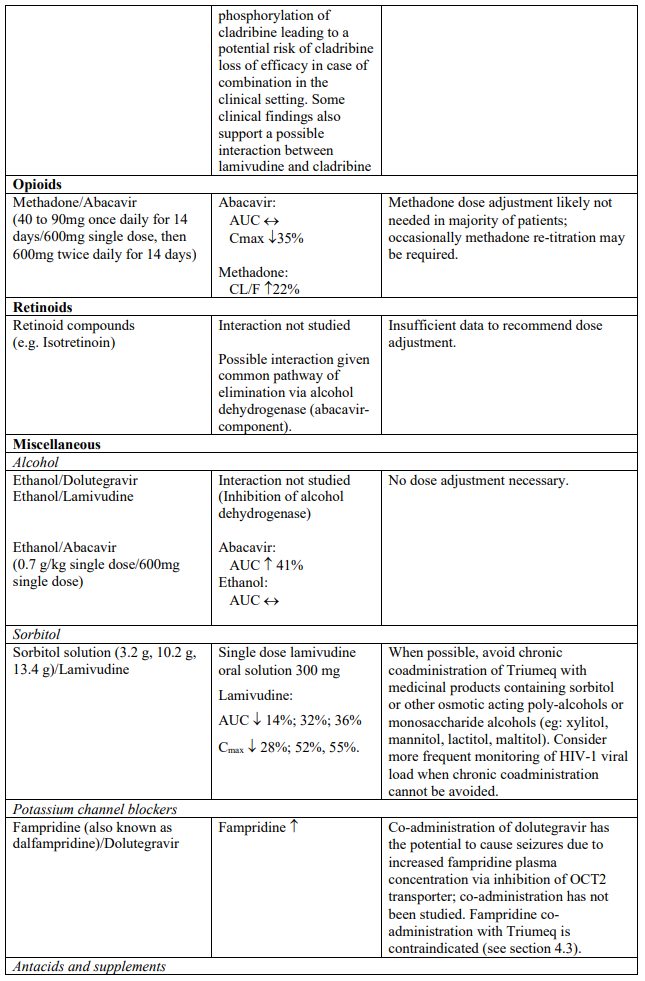
The recommended dose of dolutegravir is 50 mg twice daily when co-administered with rifampicin, carbamazepine, oxcarbazepine, phenytoin, phenobarbital, St. John’s wort, etravirine (without boosted protease inhibitors), efavirenz, nevirapine, or tipranavir/ritonavir (see section 4.5).
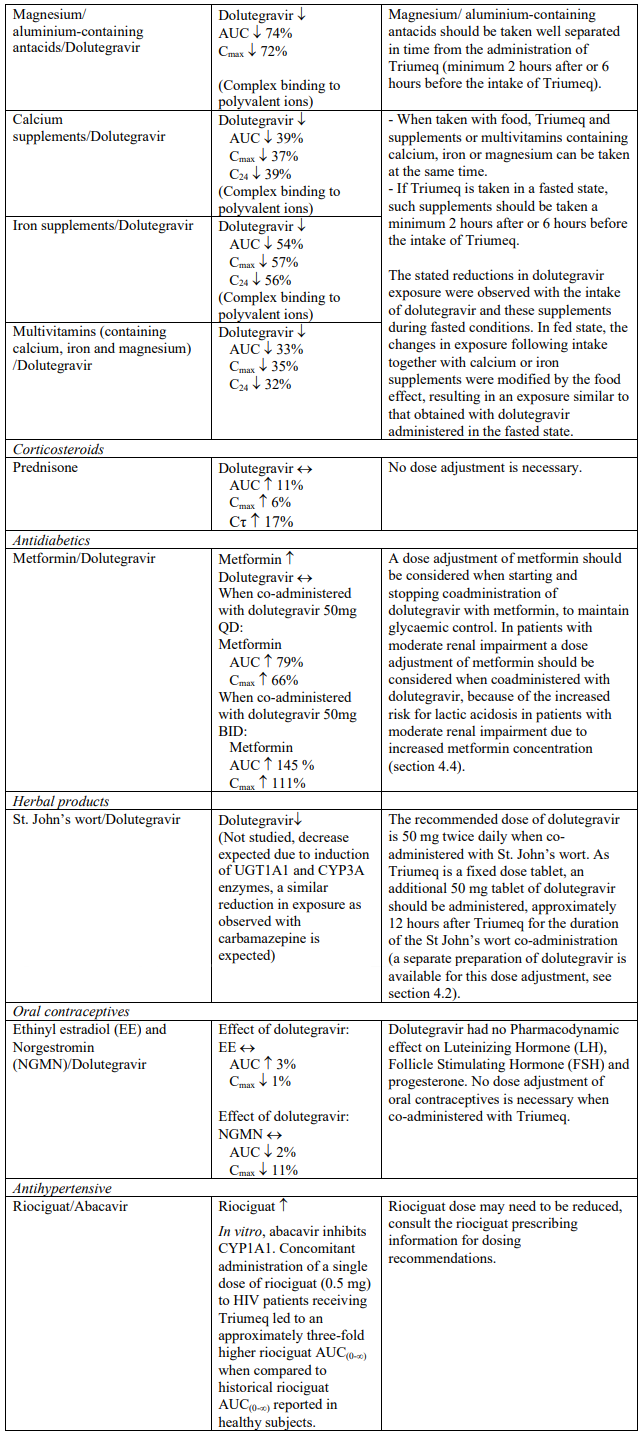
Triumeq should not be co-administered with polyvalent cation-containing antacids. Triumeq is recommended to be administered 2 hours before or 6 hours after these medicinal products (see section 4.5).
When taken with food, Triumeq and supplements or multivitamins containing calcium, iron or magnesium can be taken at the same time. If Triumeq is administered under fasting conditions, supplements or multivitamins containing calcium, iron or magnesium are recommended to be taken 2 hours after or 6 hours before Triumeq (see section 4.5).
Dolutegravir increased metformin concentrations. A dose adjustment of metformin should be considered when starting and stopping coadministration of dolutegravir with metformin, to maintain glycaemic control (see section 4.5). Metformin is eliminated renally and therefore it is of importance to monitor renal function when co-treated with dolutegravir. This combination may increase the risk for lactic acidosis in patients with moderate renal impairment (stage 3a creatinine clearance [CrCl] 45– 59 mL/min) and a cautious approach is recommended. Reduction of the metformin dose should be highly considered.
The combination of lamivudine with cladribine is not recommended (see section 4.5).
Triumeq should not be taken with any other medicinal products containing dolutegravir, abacavir, lamivudine or emtricitabine, except where a dose adjustment of dolutegravir is indicated due to drugdrug interactions (see section 4.5).
Excipients
Triumeq contains less than 1 mmol sodium (23 mg) per tablet, that is to say is essentially ‘sodium free’.
4.5. Interaction with other medicinal products and other forms of interaction
Triumeq contains dolutegravir, abacavir and lamivudine, therefore any interactions identified for these individually are relevant to Triumeq. No clinically significant drug interactions are expected between dolutegravir, abacavir and lamivudine.
Effect of other medicinal products on the pharmacokinetics of dolutegravir, abacavir and lamivudine
Dolutegravir is eliminated mainly through metabolism by uridine diphosphate glucuronosyl transferase (UGT) 1A1. Dolutegravir is also a substrate of UGT1A3, UGT1A9, CYP3A4, P-glycoprotein (P-gp), and breast cancer resistance protein (BCRP). Co-administration of Triumeq and other medicinal products that inhibit UGT1A1, UGT1A3, UGT1A9, CYP3A4, and/or P-gp may therefore increase dolutegravir plasma concentration. Medicinal products that induce those enzymes or transporters may decrease dolutegravir plasma concentration and reduce the therapeutic effect of dolutegravir (see Table 1).
The absorption of dolutegravir is reduced by certain anti-acid medicinal products (see Table 1).
Abacavir is metabolised by UGT (UGT2B7) and alcohol dehydrogenase; co-administration of inducers (e.g. rifampicin, carbamazepine and phenytoin) or inhibitors (e.g. valproic acid) of UGT enzymes or with compounds eliminated through alcohol dehydrogenase could alter abacavir exposure.
Lamivudine is cleared renally. Active renal secretion of lamivudine in the urine is mediated through the OCT2 and multidrug and toxin extrusion transporters (MATE1 and MATE2-K). Trimethoprim (an inhibitor of these drug transporters) has been shown to increase lamivudine plasma concentrations, however the resulting increase was not clinically significant (see Table 1). Dolutegravir is an OCT2 and MATE1 inhibitor; however, lamivudine concentrations were similar with or without coadministration of dolutegravir based on a cross study analysis, indicating that dolutegravir has no effect on lamivudine exposure in vivo. Lamivudine is also substrate of the hepatic uptake transporter OCT1. As hepatic elimination plays a minor role in the clearance of lamivudine, drug interactions due to inhibition of OCT1 are unlikely to be of clinical significance.
Although abacavir and lamivudine are substrates of BCRP and P-gp in vitro, given the high absolute bioavailability of abacavir and lamivudine, (see section 5.2), inhibitors of these efflux transporters are unlikely to result in a clinically relevant impact on abacavir or lamivudine concentrations.
Effect of dolutegravir, abacavir and lamivudine on the pharmacokinetics of other medicinal products
In vivo, dolutegravir did not have an effect on midazolam, a CYP3A4 probe. Based on in vivo and/or in vitro data, dolutegravir is not expected to affect the pharmacokinetics of medicinal products that are substrates of any major enzyme or transporter such as CYP3A4, CYP2C9 and P-gp (for more information see section 5.2).
In vitro, dolutegravir inhibited the renal transporters OCT2 and MATE1. In vivo, a 10-14% decrease of creatinine clearance (secretory fraction is dependent on OCT2 and MATE1 transport) was observed in patients. In vivo, dolutegravir may increase plasma concentrations of medicinal products in which excretion is dependent upon OCT2 and/or MATE1 (e.g. fampridine [also known as dalfampridine], metformin) (see Table 1).
In vitro, dolutegravir inhibited the renal uptake organic anion transporters (OAT)1 and OAT3. Based on the lack of effect on the in vivo pharmacokinetics of the OAT substrate tenofovir, in vivo inhibition of OAT1 is unlikely. Inhibition of OAT3 has not been studied in vivo. Dolutegravir may increase plasma concentrations of medicinal products in which excretion is dependent upon OAT3.
In vitro, abacavir demonstrated the potential to inhibit CYP1A1 and limited potential to inhibit metabolism mediated by CYP3A4. Abacavir was an inhibitor of MATE1; the clinical consequences are not known.
In vitro, lamivudine was an inhibitor of OCT1 and OCT2; the clinical consequences are not known.
Established and theoretical interactions with selected antiretrovirals and non-antiretroviral medicinal products are listed in Table 1.
Interaction table
Interactions between dolutegravir, abacavir, lamivudine and co-administered medical products are listed in Table 1 (increase is indicated as “↑”, decrease as “↓”, no change as “↔”, area under the concentration versus time curve as “AUC”, maximum observed concentration as "Cmax", concentration at end of dosing interval as "Cτ"). The table should not be considered exhaustive but is representative of the classes studied.
Table 1. Drug interactions:
Paediatric population
Interaction studies have only been performed in adults.
4.6. Fertility, pregnancy and lactation
Women of childbearing potential
Women of childbearing potential should be counselled about the potential risk of neural tube defects with dolutegravir (a component of Triumeq, see below), including consideration of effective contraceptive measures.
If a woman plans pregnancy, the benefits and the risks of continuing treatment with Triumeq should be discussed with the patient.
Pregnancy
Human experience from a birth outcome surveillance study in Botswana shows a small increase of neural tube defects; 7 cases in 3,591 deliveries (0.19%; 95% CI 0.09%, 0.40%) to mothers taking dolutegravir-containing regimens at the time of conception compared to 21 cases in 19,361 deliveries (0.11%: 95% CI 0.07%, 0.17%) to women exposed to non-dolutegravir regimens at the time of conception.
The incidence of neural tube defects in the general population ranges from 0.5-1 case per 1,000 live births (0.05-0.1%). Most neural tube defects occur within the first 4 weeks of embryonic development after conception (approximately 6 weeks after the last menstrual period). If a pregnancy is confirmed in the first trimester while on Triumeq, the benefits and risks of continuing Triumeq versus switching to another antiretroviral regimen should be discussed with the patient taking the gestational age and the critical time period of neural tube defect development into account.
Data analysed from the Antiretroviral Pregnancy Registry do not indicate an increased risk of major birth defects in over 600 women exposed to dolutegravir during pregnancy but are currently insufficient to address the risk of neural tube defects.
In animal reproductive toxicology studies with dolutegravir, no adverse development outcomes, including neural tube defects, were identified (see section 5.3).
More than 1000 outcomes from exposure to dolutegravir during second and third trimester pregnancy indicate no evidence of increased risk of foetal/neonatal toxicity. Triumeq may be used during the second and third trimester of pregnancy when the expected benefit justifies the potential risk to the foetus.
Dolutegravir crosses the placenta in humans. In pregnant women living with HIV, the median foetal umbilical cord concentration of dolutegravir was approximately 1.3-fold greater compared with the maternal peripheral plasma concentration.
There is insufficient information on the effects of dolutegravir on neonates.
Concerning lamivudine, a large amount of data (more than 5200 outcomes from first trimester) indicates no malformative toxicity. A moderate amount of data (more than 1200 outcomes from first trimester) indicates no malformative toxicity for abacavir.
Abacavir and lamivudine may inhibit cellular DNA replication and abacavir has been shown to be carcinogenic in animal models (see section 5.3). The clinical relevance of these findings is unknown.
Mitochondrial dysfunction
Nucleoside and nucleotide analogues have been demonstrated in vitro and in vivo to cause a variable degree of mitochondrial damage. There have been reports of mitochondrial dysfunction in HIVnegative infants exposed in utero and/or post-natally to nucleoside analogues (see section 4.4).
Breast-feeding
Dolutegravir is excreted in human milk in small amounts (a median dolutegravir breast milk to maternal plasma ratio of 0.033 has been shown). There is insufficient information on the effects of dolutegravir in neonates/infants.
Abacavir and its metabolites are excreted into the milk of lactating rats. Abacavir is also excreted into human milk.
Based on more than 200 mother/child pairs treated for HIV, serum concentrations of lamivudine in breastfed infants of mothers treated for HIV are very low (<4% of maternal serum concentrations) and progressively decrease to undetectable levels when breastfed infants reach 24 weeks of age. There are no data available on the safety of abacavir and lamivudine when administered to babies less than three months old.
It is recommended that women living with HIV do not breast-feed their infants in order to avoid transmission of HIV.
Fertility
There are no data on the effects of dolutegravir, abacavir or lamivudine on human male or female fertility. Animal studies indicate no effects of dolutegravir, abacavir or lamivudine on male or female fertility (see section 5.3).
4.7. Effects on ability to drive and use machines
Triumeq has no or negligible influence on the ability to drive and use machines. Patients should be informed that dizziness has been reported during treatment with dolutegravir. The clinical status of the patient and the adverse reaction profile of Triumeq should be borne in mind when considering the patient’s ability to drive or operate machinery.
4.8. Undesirable effects
Summary of the safety profile
The most frequently reported adverse reactions related to dolutegravir and abacavir/lamivudine were nausea (12%), insomnia (7%), dizziness (6%) and headache (6%).
Many of the adverse reactions listed in the table below occur commonly (nausea, vomiting, diarrhoea, fever, lethargy, rash) in patients with abacavir hypersensitivity. Therefore, patients with any of these symptoms should be carefully evaluated for the presence of this hypersensitivity (see section 4.4). Very rarely cases of erythema multiforme, Stevens-Johnson syndrome or toxic epidermal necrolysis have been reported where abacavir hypersensitivity could not be ruled out. In such cases medicinal products containing abacavir should be permanently discontinued.
The most severe adverse reaction related to the treatment with dolutegravir and abacavir/lamivudine, seen in individual patients, was a hypersensitivity reaction that included rash and severe liver effects (see section 4.4 and Description of selected adverse reactions in this section).
Tabulated list of adverse reactions
The adverse reactions with the components of Triumeq from clinical study and post-marketing experience are listed in Table 2 by body system, organ class and absolute frequency. Frequencies are defined as very common (≥1/10), common (≥1/100 to <1/10), uncommon (≥1/1 000 to <1/100), rare (≥1/10 000 to <1/1 000), very rare (<1/10 000).
Table 2. Tabulated list of adverse reactions associated with the combination of dolutegravir + abacavir/lamivudine in an analysis of pooled data from: Phase IIb to Phase IIIb clinical studies or postmarketing experience; and adverse reactions to treatment with dolutegravir, abacavir and lamivudine from clinical studies and post-marketing experience when used with other antiretrovirals:
| Frequency | Adverse reaction |
|---|---|
| Blood and lymphatic systems disorders | |
| Uncommon: | Neutropenia1, anaemia1, thrombocytopenia1 |
| Very rare: | pure red cell aplasia1 |
| Immune system disorders | |
| Common: | hypersensitivity (see section 4.4) |
| Uncommon: | immune reconstitution syndrome (see section 4.4) |
| Metabolism and nutrition disorders | |
| Common: | anorexia1 |
| Uncommon: | hypertriglyceridaemia, hyperglycaemia |
| Very rare: | lactic acidosis1 |
| Psychiatric disorders | |
| Very common: | insomnia |
| Common: | abnormal dreams, depression, anxiety1, nightmare, sleep disorder |
| Uncommon: | suicidal ideation or suicide attempt (particularly in patients with a pre-existing history of depression or psychiatric illness), panic attack |
| Rare: | completed suicide (particularly in patients with a pre-existing history of depression or psychiatric illness) |
| Nervous system disorders | |
| Very common: | headache |
| Common: | dizziness, somnolence, lethargy1 |
| Very rare: | peripheral neuropathy1, paraesthesia1 |
| Respiratory, thoracic and mediastinal disorders | |
| Common: | cough1, nasal symptoms1 |
| Gastrointestinal disorders | |
| Very common: | nausea, diarrhoea |
| Common: | vomiting, flatulence, abdominal pain, abdominal pain upper, abdominal distension, abdominal discomfort, gastrooesophageal reflux disease, dyspepsia |
| Rare: | pancreatitis1 |
| Hepatobiliary disorders | |
| Common: | alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) elevations |
| Uncommon: | hepatitis |
| Rare: | acute hepatic failure1, increased bilirubin2 |
| Skin and subcutaneous tissue disorders | |
| Common: | rash, pruritus, alopecia1 |
| Very rare: | erythema multiform1, Stevens-Johnson syndrome1, toxic epidermal necrolysis1 |
| Musculoskeletal and connective tissue disorders | |
| Common: | Arthralgia1, muscle disorders1 (including myalgia1) |
| Rare: | rhabdomyolysis1 |
| General disorders and administration site conditions | |
| Very common: | fatigue |
| Common: | asthenia, fever1, malaise1 |
| Investigations | |
| Common: | CPK elevations, weight increased |
| Rare: | amylase elevations1 |
1 This adverse reaction was identified from clinical studies or post-marketing experience for dolutegravir, abacavir or lamivudine when used with other antiretrovirals or postmarketing experience with Triumeq.
2 In combination with increased transaminases.
Description of selected adverse reactions
Hypersensitivity reactions
Both abacavir and dolutegravir are associated with a risk for hypersensitivity reactions (HSR), which were observed more commonly with abacavir. Hypersensitivity reaction observed for each of these medicinal products (described below) share some common features such as fever and/or rash with other symptoms indicating multi-organ involvement. Time to onset was typically 10-14 days for both abacavir and dolutegravir-associated reactions, although reactions to abacavir may occur at any time during therapy. Treatment with Triumeq must be stopped without delay if HSR cannot be ruled out on clinical grounds, and therapy with Triumeq or other abacavir or dolutegravir containing products must never be re-initiated. Please refer to section 4.4 for further details on patient management in the event of a suspected HSR to Triumeq.
Dolutegravir hypersensitivity
Symptoms have included rash, constitutional findings, and sometimes, organ dysfunction, including severe liver reactions.
Abacavir hypersensitivity
The signs and symptoms of this HSR are listed below. These have been identified either from clinical studies or post marketing surveillance. Those reported in at least 10% of patients with a hypersensitivity reaction are in bold text.
Almost all patients developing hypersensitivity reactions will have fever and/or rash (usually maculopapular or urticarial) as part of the syndrome, however reactions have occurred without rash or fever. Other key symptoms include gastrointestinal, respiratory or constitutional symptoms such as lethargy and malaise.
Skin: Rash (usually maculopapular or urticarial)
Gastrointestinal tract: Nausea, vomiting, diarrhoea, abdominal pain, mouth ulceration
Respiratory tract: Dyspnoea, cough, sore throat, adult respiratory distress syndrome, respiratory failure
Miscellaneous: Fever, lethargy, malaise, oedema, lymphadenopathy, hypotension, conjunctivitis, anaphylaxis
Neurological/Psychiatry: Headache, paraesthesia
Haematological: Lymphopenia
Liver/pancreas: Elevated liver function tests, hepatitis, hepatic failure
Musculoskeletal: Myalgia, rarely myolysis, arthralgia, elevated creatine phosphokinase
Urology: Elevated creatinine, renal failure
Symptoms related to this HSR worsen with continued therapy and can be life-threatening and in rare instance, have been fatal.
Restarting abacavir following an abacavir HSR results in a prompt return of symptoms within hours. This recurrence of the HSR is usually more severe than on initial presentation, and may include lifethreatening hypotension and death. Similar reactions have also occurred infrequently after restarting abacavir in patients who had only one of the key symptoms of hypersensitivity (see above) prior to stopping abacavir; and on very rare occasions have also been seen in patients who have restarted therapy with no preceding symptoms of a HSR (i.e., patients previously considered to be abacavir tolerant).
Metabolic parameters
Weight and levels of blood lipids and glucose may increase during antiretroviral therapy (see section 4.4).
Osteonecrosis
Cases of osteonecrosis have been reported, particularly in patients with generally acknowledged risk factors, advanced HIV disease or long-term exposure to CART. The frequency of this is unknown (see section 4.4).
Immune reactivation syndrome
In HIV-infected patients with severe immune deficiency at the time of initiation of CART, an inflammatory reaction to asymptomatic or residual opportunistic infections may arise. Autoimmune disorders (such as Graves' disease and autoimmune hepatitis) have also been reported; however, the reported time to onset is more variable and these events can occur many months after initiation of treatment (see section 4.4).
Changes in laboratory chemistries
Increases in serum creatinine occurred within the first week of treatment with dolutegravir and remained stable through 96 weeks. In the SINGLE study a mean change from baseline of 12.6 µmol/L was observed after 96 weeks of treatment. These changes are not considered to be clinically relevant since they do not reflect a change in glomerular filtration rate.
Asymptomatic creatine phosphokinase (CPK) elevations mainly in association with exercise have also been reported with dolutegravir therapy.
Co-infection with Hepatitis B or C
In dolutegravir Phase III studies patients with hepatitis B and/or C co-infection were permitted to enrol provided that baseline liver chemistry tests did not exceed 5 times the upper limit of normal (ULN). Overall, the safety profile in patients co-infected with hepatitis B and/or C was similar to that observed in patients without hepatitis B or C co-infection, although the rates of AST and ALT abnormalities were higher in the subgroup with hepatitis B and/or C co-infection for all treatment groups.
Paediatric population
There are no clinical study data on the effects of Triumeq in the paediatric population. Individual components have been investigated in infants, children and adolescents.
Based on available data with dolutegravir used in combination with other antiretroviral agents to treat infants, children and adolescents, there were no additional safety issues identified beyond those observed in the adult population.
The individual preparations of abacavir and lamivudine have been investigated separately, and as a dual nucleoside backbone, in combination antiretroviral therapy to treat ART-naive and ART-experienced HIV-infected paediatric patients (data available on the use of abacavir and lamivudine in infants less than three months are limited). No additional types of adverse reactions have been observed beyond those characterised for the adult population.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system listed in Appendix V.
6.2. Incompatibilities
Not applicable.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.