TYMLOS Solution for injection Ref.[9933] Active ingredients: Abaloparatide
Source: FDA, National Drug Code (US) Revision Year: 2020
12.1. Mechanism of Action
Abaloparatide is a PTHrP(1-34) analog which acts as an agonist at the PTH1 receptor (PTH1R). This results in activation of the cAMP signaling pathway in target cells. In rats and monkeys, abaloparatide had an anabolic effect on bone, demonstrated by increases in BMD and bone mineral content (BMC) that correlated with increases in bone strength at vertebral and/or nonvertebral sites [see Nonclinical Toxicology (13.2)].
12.2. Pharmacodynamics
Effects on Markers of Bone Turnover
A dose-finding study of abaloparatide administered once daily for 24 weeks demonstrated a dose-response relationship for BMD and bone formation markers.
Daily administration of TYMLOS to postmenopausal women with osteoporosis in clinical studies increased the bone formation marker serum procollagen type I N-propeptide (PINP). The increase in PINP levels peaked at Month 1 at 93% above baseline then decreased slowly over time. The increase in PINP was maintained above baseline throughout the treatment duration. At Month 18, PINP concentrations were approximately 45% above baseline. The increase in the bone resorption marker serum collagen type I cross-linked C-telopeptide (sCTX) peaked at Month 3 at 43% above baseline then decreased to 20% above baseline by Month 18.
Cardiac Electrophysiology
A 4-way cross-over thorough QT/QTc study was conducted in 55 healthy subjects who received single doses of placebo, subcutaneous doses of abaloparatide at 80 mcg and 240 mcg (three times the recommended dose), and moxifloxacin 400 mg orally. Abaloparatide increased heart rate, with a mean peak increase of 15 beats per minute (bpm) and 20 bpm at the first time point (15 minutes) after dosing with 80 mcg and 240 mcg, respectively. There were no clinically meaningful effects of abaloparatide on QTcI (individually corrected QT intervals) or cardiac electrophysiology.
12.3. Pharmacokinetics
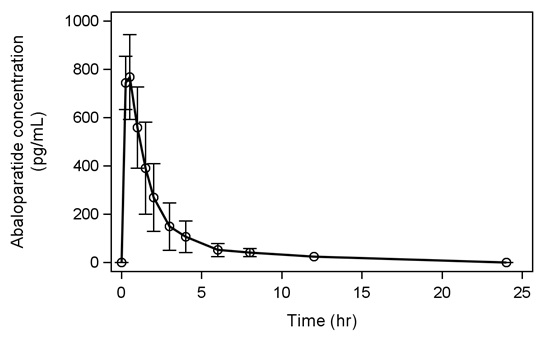
Following seven days of subcutaneous administration of abaloparatide 80 mcg, the mean (SD) abaloparatide exposure was 812 (118) pg/mL for Cmax and 1622 (641) pg·hr/mL for AUC0-24.
Figure 1 below shows the mean (SD) abaloparatide pharmacokinetic profile in postmenopausal women (N = 8) on Day 7.
Figure 1. Mean Abaloparatide Pharmacokinetic Profile in Postmenopausal Women on Day 7:
Absorption
The median (range) time to peak concentration of abaloparatide 80 mcg was 0.51 hr (0.25 to 0.52 hr) following subcutaneous administration. The absolute bioavailability of abaloparatide in healthy women after subcutaneous administration of an 80 mcg dose was 36%.
Distribution
The in vitro plasma protein binding of abaloparatide was approximately 70%. The volume of distribution was approximately 50 L.
Elimination
The mean (SD) half-life of abaloparatide is 1.7 (0.7) hrs. The peptide fragments are primarily eliminated through renal excretion.
Metabolism
No specific metabolism or excretion studies have been performed with TYMLOS. The metabolism of abaloparatide is consistent with non-specific proteolytic degradation into smaller peptide fragments, followed by elimination by renal clearance.
Specific Populations
Geriatric Patients
No age-related differences in abaloparatide pharmacokinetics were observed in postmenopausal women ranging from 49 to 86 years of age.
Race
No differences in abaloparatide pharmacokinetics based on race were observed in clinical trials.
Patients with Renal Impairment
A single 80 mcg subcutaneous dose of abaloparatide was administered to male and female patients with renal impairment: 8 patients with mild renal impairment (CLCr 60 to 89 mL/min), 7 patients with moderate renal impairment (CLCr 30 to 59 mL/min), 8 patients with severe renal impairment (CLCr 15 to 29 mL/min), and 8 healthy subjects with normal renal function (CLCr 90 or greater mL/min) matched by sex, age, and body mass index (BMI). Abaloparatide Cmax increased 1.0-, 1.3-, and 1.4-fold in patients with mild, moderate, and severe renal impairment, compared to the healthy subjects with normal renal function. Abaloparatide AUC increased 1.2-, 1.7-, and 2.1-fold in patients with mild, moderate, and severe renal impairment, compared to the healthy subjects with normal renal function. Patients undergoing dialysis were not included in the study.
Drug Interactions
In vitro studies showed that abaloparatide, at therapeutic concentrations, does not inhibit or induce Cytochrome P450 enzymes.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 2-year carcinogenicity study, abaloparatide was administered once daily to male and female Fischer rats by subcutaneous injection at doses of 10, 25, and 50 mcg/kg. These doses resulted in systemic exposures to abaloparatide that were 4, 16, and 28 times, respectively, the systemic exposure observed in humans following the recommended subcutaneous dose of 80 mcg (based on AUC comparisons). Neoplastic changes related to treatment with abaloparatide consisted of marked dose-dependent increases in osteosarcoma and osteoblastoma incidence in all male and female dose groups. The incidence of osteosarcoma was 0-2% in untreated controls and reached 87% and 62% in male and female high-dose groups, respectively. The bone neoplasms were accompanied by marked increases in bone mass.
The relevance of the rat findings to humans is uncertain. The use of TYMLOS is not recommended in patients at increased risk of osteosarcoma [see Warnings and Precautions (5.1)].
Abaloparatide was not genotoxic or mutagenic in a standard battery of tests including the Ames test for bacterial mutagenesis, the chromosome aberration test using human peripheral lymphocytes, and the mouse micronucleus test.
13.2. Animal Toxicology and/or Pharmacology
In toxicity studies in rats and monkeys of up to 26-week and 39-week duration, respectively, findings included vasodilation, increases in serum calcium, decreases in serum phosphorus, and soft tissue mineralization at doses ≥10 mcg/kg/day. The 10 mcg/kg/day dose resulted in systemic exposures to abaloparatide in rats and monkeys that were 2 and 3 times, respectively, the exposure in humans at daily subcutaneous doses of 80 mcg.
Pharmacologic effects of abaloparatide on the skeleton were assessed in 12- and 16-month studies in ovariectomized (OVX) rats and monkeys, at doses up to 11 and 1 times human exposure at the recommended subcutaneous dose of 80 mcg, respectively (based on AUC comparisons). In these animal models of postmenopausal osteoporosis, treatment with abaloparatide resulted in dose-dependent increases in bone mass at vertebral and/or nonvertebral sites, correlating with increases in bone strength. The anabolic effect of abaloparatide was due to the predominant increase in osteoblastic bone formation and was evidenced by increases in trabecular thickness and/or cortical thickness due to endosteal bone apposition. Abaloparatide maintained or improved bone quality at all skeletal sites evaluated and did not cause any mineralization defects.
14. Clinical Studies
Efficacy Study in Women with Postmenopausal Osteoporosis
The efficacy of TYMLOS for the treatment of postmenopausal osteoporosis was evaluated in Study 003 (NCT 01343004), an 18-month, randomized, multicenter, double-blind, placebo-controlled clinical trial in postmenopausal women aged 49 to 86 years (mean age of 69) who were randomized to receive TYMLOS 80 mcg (N = 824) or placebo (N = 821) given subcutaneously once daily. Approximately 80% of patients were Caucasian, 16% were Asian, and 3% were Black; 24% were Hispanic. At baseline, the mean T-scores were -2.9 at the lumbar spine, -2.1 at the femoral neck, and -1.9 at the total hip. At baseline, 24% of patients had at least one prevalent vertebral fracture and 48% had at least one prior nonvertebral fracture. Patients took daily supplemental calcium (500 to 1000 mg) and vitamin D (400 to 800 IU).
The efficacy study was extended as Study 005 (NCT 01657162), an open-label study where patients were no longer receiving TYMLOS or placebo but were maintained in their original randomized treatment group and received 70 mg alendronate weekly, with calcium and vitamin D supplements for 6 months. Study 005 enrolled 1139 patients, representing 92% of patients who completed Study 003. This included 558 patients who had previously received TYMLOS and 581 patients who had previously received placebo. The cumulative 25-month efficacy dataset included 18 months of exposure to TYMLOS or placebo in Study 003, 1 month of no treatment, followed by 6 months of alendronate therapy in Study 005. Study 005 was then continued to complete 18 months of additional alendronate exposure during which time patients were no longer blinded to their original Study 003 treatment group.
Effect on New Vertebral Fractures
The primary endpoint was the incidence of new vertebral fractures in patients treated with TYMLOS compared to placebo. TYMLOS resulted in a significant reduction in the incidence of new vertebral fractures compared to placebo at 18 months (0.6% TYMLOS compared to 4.2% placebo, p <0.0001). The absolute risk reduction in new vertebral fractures was 3.6% at 18 months and the relative risk reduction was 86% for TYMLOS compared to placebo ( Table 2). The incidence of new vertebral fractures at 25 months was 0.6% in patients treated with TYMLOS then alendronate, compared to 4.4% in patients treated with placebo then alendronate (p ˂0.0001). The relative risk reduction in new vertebral fractures at 25 months was 87% for patients treated with TYMLOS then alendronate, compared to patients treated with placebo then alendronate, and the absolute risk reduction was 3.9% ( Table 2). After 24 months of open-label alendronate therapy, the vertebral fracture risk reduction achieved with TYMLOS therapy was maintained.
Table 2. Percentage of Postmenopausal Women with Osteoporosis with New Vertebral Fractures (modified Intent to Treat population)* †:
| Percentage of Postmenopausal Women With Fractures | Absolute Risk Reduction ()(95 CI‡) | Relative Risk Reduction ()(95 CI‡) | ||
|---|---|---|---|---|
| TYMLOS (N=690*)(%) | Placebo (N=711*)(%) | |||
| 0-18 months | 0.6 | 4.2 | 3.6 (2.1, 5.4) | 86 (61, 95) |
| TYMLOS/ Alendronate (N=544†)(%) | Placebo/Alendronate (N=568†)(%) | |||
| 0-25 months | 0.6 | 4.4 | 3.9 (2.1, 5.9) | 87 (59, 96) |
* Includes patients who had both pre- and post-treatment spine radiographs in Study 003
† Includes patients who had both pre- and post-treatment spine radiographs in Study 005
‡ Confidence Interval
Effect on Nonvertebral Fractures
TYMLOS resulted in a significant reduction in the incidence of nonvertebral fractures at the end of the 18 months of treatment plus 1 month follow-up where no drug was administered (2.7% for TYMLOS-treated patients compared to 4.7% for placebo-treated patients). The relative risk reduction in nonvertebral fractures for TYMLOS compared to placebo was 43% (logrank test p = 0.049) and the absolute risk reduction was 2.0%.
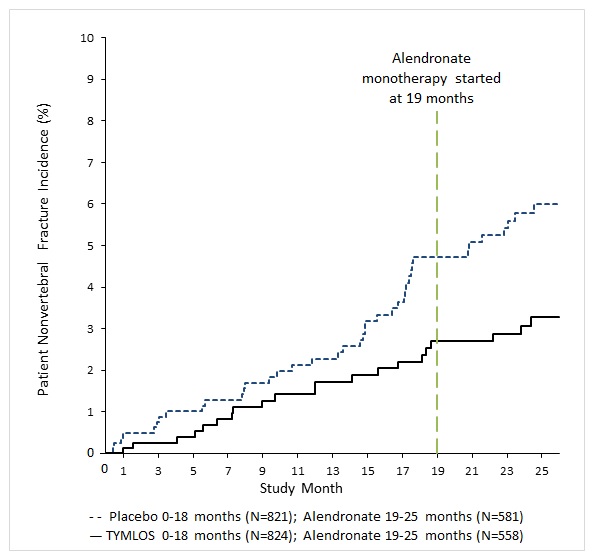
Following 6 months of alendronate treatment in Study 005, the cumulative incidence of nonvertebral fractures at 25 months was 2.7% for women in the prior TYMLOS group compared to 5.6% for women in the prior placebo group ( Figure 2). At 25 months, the relative risk reduction in nonvertebral fractures was 52% (logrank test p = 0.017) and the absolute risk reduction was 2.9%.
Figure 2. Cumulative Incidence of Nonvertebral Fractures* Over 25 Months (Intent to Treat Population)†
* Excludes fractures of the sternum, patella, toes, fingers, skull and face and those associated with high trauma.
† Includes patients randomized in Study 003
TYMLOS demonstrated consistent reductions in the risk of vertebral and nonvertebral fractures regardless of age, years since menopause, presence or absence of prior fracture (vertebral, nonvertebral) and BMD at baseline.
Effect on Bone Mineral Density (BMD)
Treatment with TYMLOS for 18 months in Study 003 resulted in significant increases in BMD compared to placebo at the lumbar spine, total hip and femoral neck, each with p<0.0001 ( Table 3). Similar findings were seen following 6 months of alendronate treatment in Study 005 ( Table 3).
Table 3: Mean Percent Changes in Bone Mineral Density (BMD) From Baseline to Endpoint in Postmenopausal Women with Osteoporosis (Intent to Treat Population)*†‡
| TYMLOS (N=824*) (%) | Placebo (N=821*) (%) | Treatment Difference () (95 CI§) | |
|---|---|---|---|
| 18 Months | |||
| Lumbar Spine | 9.2 | 0.5 | 8.8 (8.2, 9.3) |
| Total Hip | 3.4 | -0.1 | 3.5 (3.3, 3.8) |
| Femoral Neck | 2.9 | -0.4 | 3.3 (3.0, 3.7) |
| TYMLOS/Alendronate (N=558†) (%) | Placebo/Alendronate (N=581†)(%) | ||
| 25 Months | |||
| Lumbar Spine | 12.8 | 3.5 | 9.3 (8.6, 10.1) |
| Total Hip | 5.5 | 1.4 | 4.1 (3.7, 4.5) |
| Femoral Neck | 4.5 | 0.5 | 4.1 (3.6, 4.6) |
* Includes patients randomized in Study 003
† Includes patients enrolled in Study 005
‡Last-observation-carried-forward
§Confidence Interval
TYMLOS demonstrated consistent increases in BMD regardless of age, years since menopause, race, ethnicity, geographic region, presence or absence of prior fracture (vertebral, nonvertebral), and BMD at baseline.
Effect on Bone Histology
Bone biopsy specimens were obtained from 71 patients with osteoporosis after 12-18 months of treatment (36 in the TYMLOS group and 35 in the placebo group). Of the biopsies obtained, 55 were adequate for quantitative histomorphometry assessment (27 in the TYMLOS group and 28 in the placebo group). Qualitative and quantitative histology assessment showed normal bone architecture and no evidence of woven bone, marrow fibrosis, or mineralization defects.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.