UPSTAZA Solution for infusion Ref.[50861] Active ingredients: Eladocagene exuparvovec
Source: European Medicines Agency (EU) Revision Year: 2023 Publisher: PTC Therapeutics International Limited, 70 Sir John Rogerson's Quay, Dublin 2, Ireland
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Other alimentary tract and metabolism products, Enzymes
ATC code: A16AB26
Mechanism of action
AADC deficiency is an inborn error of neurotransmitter biosynthesis with an autosomal recessive inheritance in the dopa decarboxylase (DDC) gene. The DDC gene encodes the AADC enzyme, which converts L-3,4-dihydroxyphenylalanine (L-DOPA) to dopamine. Mutations in the DDC gene result in reduction or absence of AADC enzyme activity, causing a reduction in the levels of dopamine and the failure of most patients with AADC deficiency to achieve developmental milestones.
Eladocagene exuparvovec is a gene therapy based on recombinant AAV2 vector containing the human cDNA for the DDC gene. After infusion into the putamen, the product results in the expression of the AADC enzyme and subsequent production of dopamine, and consequently, development of motor function in treated AADC-deficient patients.
Pharmacodynamic effects
L-6-[18F] fluoro-3, 4-dihydroxyphenylalanine (18F-DOPA) uptake in central nervous system (CNS)
Measurement of 18F-DOPA uptake in the putamen via positron emission tomography (PET) imaging following treatment is an objective measurement of de novo dopamine production in the brain and assesses the success and stability of the AADC gene transduction over time. Most patients demonstrated small sustained increases in PET-specific uptake. An increase was evident as early as 6 months, was further increased by 12 months after treatment, and sustained at least for 5 years.
Table 4. PET specific uptake after eladocagene exuparvovec treatment (Studies AADC010, AADC-011):
| Timepoint | Baseline (n=20) | Change from baseline Month 12 (n=17) | Change from baseline Month 24 (n=15) | Change from baseline Month 60 (n=4) |
|---|---|---|---|---|
| PET specific uptake | 0.27 | 0.32 | 0.36 | 0.39 |
Clinical efficacy and safety
The efficacy of Upstaza gene therapy was assessed in 2 clinical studies (AADC-010, AADC-011). Together, these 2 studies included 20 patients with severe AADC deficiency, diagnosed by decreased homovanillic acid and 5-hydroxyindoleacetic acid and elevated L-DOPA CSF levels, the presence of DDC gene mutation in both alleles, and the presence of clinical symptoms of AADC deficiency (including developmental delay, hypotonia, dystonia, and oculogyric crisis [OGC]). These patients had not achieved motor development milestones at baseline including the ability to sit, stand or walk, compatible with the severe phenotype. Patients were treated with a total dose of 1.8 × 1011 vg (N=13) or 2.4 × 1011 vg (N=7) during a single operative session. The results for efficacy and safety parameters were similar between the 2 doses.
Study AADC-CU/1601 was conducted with treatment from an older manufacturing process. This study enrolled 8 subjects and demonstrated similar results with benefits maintained up to 60 months.
Motor function
Motor milestone achievement was derived from the Peabody Developmental Motor Scale, version 2 (PDMS-2). The PDMS-2 is an assessment of a child's motor development up to the developmental age of 5, and assesses both gross and fine motor skills, and with items that specifically capture motor milestone achievement. The PDMS-2 motor skill items were chosen to determine the number of patients who achieved at least the following motor milestones: 1) full head control, 2) sitting unassisted, 3) standing with support, and 4) walking assisted.
Table 5 summarises patient motor milestone achievement at specific timepoints during the first 60 months following treatment administration and cumulatively throughout the entire clinical programme. The primary efficacy endpoint was assessed at 24 months after gene therapy. Not all subjects reached the timepoints specified in the Table 5 at the time of data cut. Treatment with eladocagene exuparvovec demonstrated acquisition of motor milestones observed as early as 12 months post-surgery. Key motor milestone acquisition was continued or maintained beyond 24 months and up to 60 months.
Table 5. Number of patients achieving new PDMS-2 motor milestones (mastery of the skill – score 2) after eladocagene exuparvovec treatment (Studies AADC-010, AADC-011):
| Baseline | Time interval post-treatment (months) | Overall (cumulative) post- treatment | ||||||
|---|---|---|---|---|---|---|---|---|
| Motor milestone | Pre- treatment N=20 | 0 to 3 N=20 | 3 to 12 N=17 | 12 to 24 N=17 | 24 to 36 N=13 | 36 to 48 N=8 | 48 to 60 N=6 | 60 months N=20 |
| Head control | 0 | 1 | 5 | 6 | 2 | 0 | 0 | 14 (70%) |
| Sitting unassisted | 0 | 1 | 2 | 6 | 2 | 1 | 1 | 13 (65%) |
| Standing with support | 0 | 0 | 0 | 4 | 1 | 1 | 0 | 6 (30%) |
| Walking with support | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 2 (10%) |
Note: Cumulative column includes all subjects who achieved that particular milestone at any point during the clinical study up to 60 months; Patients needed to reach the score of 2 (indicative of mastery of the skill) on a milestone item to be rated as having achieved that milestone.
PDMS-2 total score
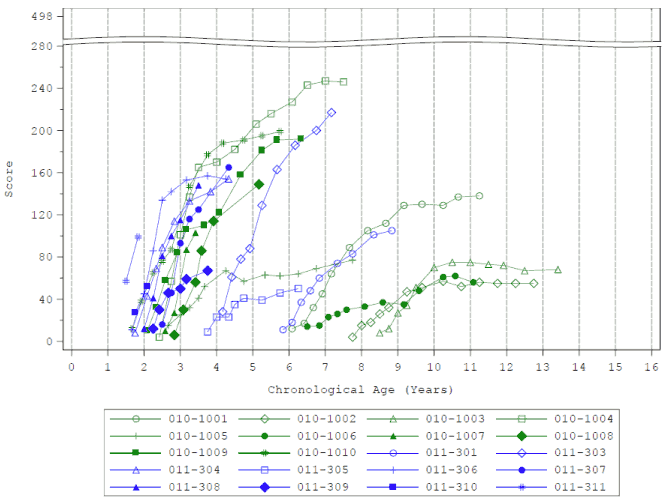
PDMS-2 total score was measured as a secondary endpoint throughout the clinical studies. PDMS-2 maximal scores are 450-482, depending on age (<12 months or >12 months). All subjects treated with eladocagene exuparvovec showed increases from baseline in mean PDMS-2 total scores over time, with some benefit observed as early as 3 months (Figure 2). At the 24-month timepoint, the least squares (LS) mean of change from baseline in PDMS-2 total score was 104.4 points. Improvement from baseline in PDMS-2 total score was as early as 12 months after treatment (76.1 points) and was maintained to 60 months (108.2 points). Patients who receive eladocagene exuparvovec at a younger age demonstrate a faster treatment response and appear to reach a higher final level.
Figure 2. Mean PDMS-2 total scores by visit – through month 60 (Studies AADC-010, AADC-011):
The following data were collected as secondary endpoints in the clinical studies.
Cognitive and communication skills
The total language score, subscales of Bayley-III, a standard assessment of cognition, language, and motor development for, infants and toddlers (1-42 months of age) was assessed in Studies AADC-10 and AADC-11. Over time, all subjects showed gradual and sustained increases in mean total language score, which is the combined score for receptive and expressive communication subscales. The total score of the language subscale is 97. The mean at baseline was 17.70 (N=20). The mean change from baseline for total language score was 7.35 at Month 12 (N=17), 9.87 at Month 24 (N=15), and 12.60 at Month 36 (N=10).
Body weight
Sixteen out of 17 subjects (94%) maintained (47%, 8 subjects) or increased (47%, 8 subjects) their body weight over a 12-month period based on gender and age specific growth chart.
Floppiness (hypotonia) limb dystonia, stimulus-provoked dystonia
Following gene therapy, the percentage of subjects with symptoms of floppiness (hypotonia) decreased from 77.8% at baseline (N=20) to 46.7% at Month 12 (N=17). No subject experienced limb dystonia and stimulus-provoked dystonia 12 months post-treatment, compared with 66.7% and 11.1% subjects at baseline (N=20), respectively.
OGC episodes
Following gene therapy, the duration of OGC episodes, was reduced and sustained over time and up to 12 months after treatment. The mean time in OGC was 12.30 hours/week at baseline. This time was reduced following treatment by 1.85 hours per week by Month 3 (N=16) and by 3.66 hours per week by Month 12 (N=6).
The magnitude of the effect of eladocagene exuparvovec on the autonomic symptoms of the AADC deficiency has not been systematically evaluated.
Exceptional circumstances
This medicinal product has been authorised under 'exceptional circumstances'. This means that due to the rarity of the disease it has not been possible to obtain complete information on this medicinal product. The European Medicines Agency will review any new information which may become available every year and this SmPC will be updated as necessary.
5.2. Pharmacokinetic properties
No pharmacokinetic studies with eladocagene exuparvovec have been conducted. Eladocagene exuparvovec is infused directly into the brain and has not been shown to distribute outside the CNS.
Distribution
The biodistribution of the AAV2-hAADC viral vector in blood and urine was measured in subjects using a validated real-time quantitative polymerase chain reaction assay. Subjects treated with Upstaza showed no evidence of detectable viral vector in blood or urine at baseline or through 12 months after treatment.
5.3. Preclinical safety data
No animal studies have been conducted to evaluate the effects of eladocagene exuparvovec on carcinogenesis, mutagenesis and impairment of fertility. In animal studies, no toxicological effects on male or female reproductive organs were observed.
No toxicity was shown in rats up to 6 months following bilateral infusion into the putamen at doses 21 times higher than the human therapeutic dose on a vg per unit of brain weight (g) basis. Studies in rats showed no viral shedding in blood or any systemic tissues outside of the CNS compartment except for CSF at day 7 where it was positive (copies/µg DNA) in the 6-month toxicology study. When tested at subsequent time points (day 30, day 90 and day 180) all samples were negative.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.