UPSTAZA Solution for infusion Ref.[50861] Active ingredients: Eladocagene exuparvovec
Source: European Medicines Agency (EU) Revision Year: 2023 Publisher: PTC Therapeutics International Limited, 70 Sir John Rogerson's Quay, Dublin 2, Ireland
4.1. Therapeutic indications
Upstaza is indicated for the treatment of patients aged 18 months and older with a clinical, molecular, and genetically confirmed diagnosis of aromatic L-amino acid decarboxylase (AADC) deficiency with a severe phenotype (see section 5.1).
4.2. Posology and method of administration
Treatment should be administered in a centre which is specialised in stereotactic neurosurgery, by a qualified neurosurgeon under controlled aseptic conditions.
Posology
Patients will receive a total dose of 1.8 × 1011 vg delivered as four 0.08 mL (0.45 × 1011 vg) infusions (two per putamen).
The posology is the same for the entire population covered by the indication.
Special populations
Paediatric population
The safety and efficacy of eladocagene exuparvovec in children aged below 18 months have not yet been established. No data are available. There is limited experience in patients aged 12 years and older. The safety and efficacy of eladocagene exuparvovec in these patients have not been established. Currently available data are described in section 5.1. No dose adjustment should be considered.
Hepatic and renal impairment
The safety and efficacy of eladocagene exuparvovec in patients with hepatic and renal impairment have not been evaluated.
Immunogenicity
There is no safety or efficacy data for patients whose pre-treatment neutralising antibody levels to AAV2 was >1:20 (see section 4.4).
Method of administration
Intraputaminal use.
Preparation
Upstaza is a sterile solution for infusion that requires thawing and preparation by the hospital pharmacy prior to administration.
For detailed instructions on preparation, administration, measures to take in case of accidental exposure and disposal of Upstaza, see section 6.6.
Neurosurgical administration
Upstaza is a single use vial administered by bilateral intraputaminal infusion in one surgical session at two sites per putamen. Four separate infusions of equal volumes are performed to the right anterior putamen, right posterior putamen, left anterior putamen, and left posterior putamen. For instructions on preparation of the surgical suite infusion of Upstaza, see section 6.6.
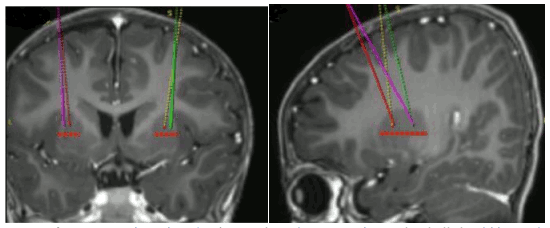
The target infusion sites are defined per standard stereotactic neurosurgical practice. Upstaza is administered as a bilateral infusion (2 infusions per putamen) with an intracranial cannula. The final 4 targets for each trajectory should be defined as 2 mm dorsal to (above) the anterior and posterior target points in the mid-horizonal plane (Figure 1).
Figure 1. Four target points for infusion sites:
- After stereotactic registration is complete, the entry point on the skull should be marked. Surgical access through the skull bone and dura should be performed.
- The infusion cannula is placed at the designation point in the putamen using stereotactic tools based on the trajectories planned. Of note, the infusion cannula is placed and infusion performed separately for each putamen.
- Upstaza is infused at a rate of 0.003 mL/min at each of the 2 target points in each putamen; 0.08 mL of Upstaza is infused per putaminal site resulting in 4 infusions with a total volume of 0.320 mL (or 1.8 × 1011 vg).
- Starting with the first target site, the cannula is inserted through a burr hole into the putamen and then slowly withdrawn, distributing the 0.08 mL of Upstaza across the planned trajectory to optimise distribution across the putamen.
- After the first infusion, the cannula is withdrawn and then re-inserted at the next target point, repeating the same procedure for the other 3 target points (anterior and posterior of each putamen).
- After standard neurosurgical closure procedures, the patient then undergoes a postoperative computerised tomography imaging examination to ensure there are no complications (ie, bleeding).
- The patient must reside within the vicinity of the hospital where the procedure was performed for a minimum of 48 hours following the procedure. The patient may return home, post-procedure, based on treating physician's advice. The post-treatment care should be managed by the referring paediatric neurologist and with the neurosurgeon. The patient should have a follow-up 7 days after surgery to ensure that no complications have developed. A second follow-up visit should take place 2 weeks later (ie, 3 weeks after the surgery) to monitor post-surgical recovery and occurrence of adverse events.
- Patients will be offered to enrol in a registry in order to further evaluate the long-term safety and effectiveness of the treatment under normal conditions of clinical practice.
4.9. Overdose
There is no clinical experience with overdose of eladocagene exuparvovec. Symptomatic and supportive treatment, as deemed necessary by the treating physician, is advised in case of overdose. Close clinical observation and monitoring of laboratory parameters (including complete blood count with differential, and comprehensive metabolic panel) for systemic immune response are recommended. For instructions in case of accidental exposure, see section 6.6.
6.3. Shelf life
Unopened frozen vial:
42 months.
After thawing and opening:
Once thawed, the medicinal product should not be re-frozen. The filled syringe prepared under aseptic conditions for delivery to the surgical site should be used immediately; if not used immediately, it can be stored at room temperature (below 25°C) and used within 6 hours of starting product thaw.
6.4. Special precautions for storage
Store and transport frozen at ≤ -65°C.
Keep the vial in the outer carton.
For storage conditions after thawing and opening of the medicinal product, see section 6.3.
6.5. Nature and contents of container
Type I borosilicate glass vial, with a siliconised chlorobutyl stopper with coating sealed with an aluminium/plastic cap.
Pack size of one vial.
6.6. Special precautions for disposal and other handling
Each vial is for single use only. This medicinal product should only be infused with the SmartFlow ventricular cannula.
Precautions to be taken before handling or administering the medicinal product:
This medicinal product contains genetically modified virus. During preparation, administration, and disposal, personal protective equipment (including gown, safety glasses, mask, and gloves) should be worn when handling eladocagene exuparvovec and materials that have been in contact with the solution (solid and liquid waste).
Thawing in the hospital pharmacy:
- Upstaza is delivered to the pharmacy frozen and must be maintained in the outer carton at ≤ -65ºC until prepared for use.
- Upstaza should be handled aseptically under sterile conditions.
- Allow the frozen vial of Upstaza to thaw upright at room temperature until the content is completely thawed. Gently invert the vial approximately 3 times, do NOT shake.
- Inspect Upstaza after mixing. If particulates, cloudiness, or discolouration are visible, do not use the product.
Preparation prior to administration:
- Transfer the vial, syringe, needle, syringe cap, sterile bags, or sterile wrappings compliant with hospital procedure for transfer and use of the filled syringe in the planned surgical suite, and label into the Biological Safety Cabinet (BSC). Wear sterile gloves and other personal protective equipment (including gown, safety glasses and mask) as per normal procedure for BSC work.
- Open the 5-mL syringe [5 mL, polypropylene syringes with latex-free elastomer plunger, lubricated with medical-grade silicone oil] and label as the product-filled syringe per pharmacy procedure and local regulations.
- Attach the 18-or 19-gauge filter needle [18- or 19-gauge, 1.5-inch, stainless steel, 5-µm filter needles] to the syringe.
- Draw the full volume of the vial of Upstaza into the syringe. Invert the vial and syringe and partially withdraw or angle the needle as necessary to maximise recovery of product.
- Draw air in the syringe so that the needle is emptied of product. Carefully remove the needle from 5-mL syringe containing Upstaza. Purge the air from the syringe until there is no air bubble and then cap with a syringe cap.
- Wrap the syringe in one sterile plastic bag (or several bags based on standard hospital procedure) and place in an appropriate secondary container (eg, hard plastic cooler) for delivery to the surgical suite at room temperature. Use of the syringe (ie, connecting the syringe to the syringe pump and starting priming of the cannula) should begin within 6 hours of starting product thaw.
Administration in the surgical suite:
- Tightly connect the syringe containing Upstaza to the SmartFlow ventricular cannula.
- Install the Upstaza syringe into a syringe infusion pump compatible with the 5-mL syringe. Pump Upstaza with the infusion pump at 0.003 mL/min until the first drop of Upstaza can be seen from the tip of the needle. Stop and wait until ready for infusion.
Precautions to be taken for the disposal of the medicinal product and accidental exposure:
- Accidental exposure to eladocagene exuparvovec, including contact with skin, eyes, and mucous membranes, is to be avoided.
- In the event of exposure to skin, the affected area must be thoroughly cleaned with soap and water for at least 5 minutes. In the event of exposure to eyes, the affected area must be thoroughly flushed with water for at least 5 minutes.
- In the event of needlestick injury, the affected area must be cleaned thoroughly with soap and water and/or a disinfectant.
- Any unused eladocagene exuparvovec or waste material should be disposed of in compliance with local guidance for pharmaceutical waste. Potential spills should be wiped with absorbent gauze and disinfected using a bleach solution followed by alcohol wipes.
- After administration, the risk of shedding is considered to be low. It is recommended that caregivers and patient families are advised on and follow proper handling precautions of patient bodily fluids and waste for 14 days after administration of eladocagene exuparvovec (see section 4.4).
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.