URITOS / URITOS OD Tablet Ref.[50721] Active ingredients: Imidafenacin
Source: Web Search Revision Year: 2017
Product description
| Tablets 0.1 mg | OD Tablets 0.1 mg | ||
|---|---|---|---|
| Active ingredient Content per tablet | Imidafenacin 0.1 mg | Imidafenacin 0.1 mg | |
| Inactive ingredients | Microcrystalline cellulose, Partly pregelatinized starch, Povidone, Magnesium stearate, Hypromellose, Titanium oxide, Red ferric oxide, Carnauba wax | Partly pregelatinized starch, Aminoalkyl methacrylate copolymer E, Magnesium stearate, D-Mannitol, Crospovidone, Hydrated silicon dioxide | |
| Type of tablet | Film-coated tablets | Plain tablets (orally disintegrating tablets) | |
| Color | Pale red to reddish brown or pale reddish violet | White | |
| Size | Diameter | 7.1 mm | 7.6 mm |
| Thickness | 3.5 mm | 4.1 mm | |
| Weight | 140 mg | 180 mg | |
| Identification code | URITOS 0.1 (tablet) KP-197 (package) | KP-121 | |
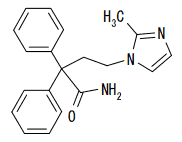
Nonproprietary name: Imidafenacin (JAN)
Chemical name: 4-(2-Methyl-1H-imidazol-1-yl)-2,2-diphenylbutanamide
Molecular formula: C20H21N3O
Molecular weight: 319.40
Melting point: 192 to 196°C
Description: Imidafenacin occurs as a white crystal or crystalline powder. It is freely soluble in acetic acid (100), soluble in N,N-dimethyl formamide (DMF) and methanol, sparingly soluble in ethanol (99.5), slightly soluble in acetonitrile, and practically insoluble in water.
Partition coefficient:
| Organic phase | Aqueous phase | Partition coefficient |
|---|---|---|
| 1-Octanol | pH4.03 (McIlvaine’s buffer) | 0.0664 |
| 1-Octanol | pH6.08 (McIlvaine’s buffer) | 4.47 |
| 1-Octanol | pH8.07 (McIlvaine’s buffer) | 240 |
| How Supplied |
|---|
|
URITOS Tablets 0.1 mg: PTP package: 100 tablets (10 tablets × 10) Non-sealed package: 500 tablets URITOS OD Tablets 0.1 mg: PTP package: 100 tablets (10 tablets × 10) Storage conditions: Protect from moisture after opening aluminum package. Manufactured and marketed by: Kyorin Pharmaceutical Co., Ltd. 6, Kanda surugadai 4-chome, Chiyoda-ku, Tokyo 101-8311, Japan |
Drugs
| Drug | Countries | |
|---|---|---|
| URITOS | Ecuador, Japan |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.