VARILRIX Powder and solvent for solution for injection Ref.[8243] Active ingredients: Varicella, live attenuated
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2021 Publisher: SmithKline Beecham Ltd, 980, Great West Road, Brentford, Middlesex TW8 9GS, United Kingdom Trading as: GlaxoSmithKline UK
Therapeutic indications
Varilrix is indicated for active immunisation against varicella:
- In healthy individuals from 9 to 11 months of age (see section 5.1), under special circumstances;
- In healthy individuals from the age of 12 months (see section 5.1);
- For post-exposure prophylaxis if administered to healthy, susceptible individuals exposed to varicella within 72 hours of contact (see sections 4.4 and 5.1);
- In individuals at high risk of severe varicella (see sections 4.3, 4.4 and 5.1).
The use of Varilrix should be based on official recommendations.
Posology and method of administration
Posology
The immunisation schedules for Varilrix should be based on official recommendations.
Healthy individuals
Infants from 9 months to 11 months of age (inclusive)
Infants from 9 to 11 months of age (inclusive) receive two doses of Varilrix to ensure optimal protection against varicella (see section 5.1). The second dose should be given after a minimum interval of 3 months.
Children from 12 months of age, adolescents and adults
Children from the age of 12 months as well as adolescents and adults receive two doses of Varilrix to ensure optimal protection against varicella (see section 5.1). The second dose should generally be given at least 6 weeks after the first dose. Under no circumstances should the interval between the doses be less than 4 weeks.
Individuals at high risk of severe varicella
Individuals at high risk of severe varicella may benefit from re-vaccination following the 2-dose schedule (see section 5.1). Periodic measurement of varicella antibodies after immunisation may be indicated in order to identify those who may benefit from re-immunisation. Under no circumstances should the interval between the doses be less than 4 weeks.
Other paediatric population
The safety and efficacy of Varilrix in infants less than 9 months of age have not yet been established. No data are available.
Interchangeability
A single dose of Varilrix may be administered to those who have already received a single dose of another varicella-containing vaccine.
A single dose of Varilrix may be administered followed by a single dose of another varicella-containing vaccine.
Method of administration
Varilrix is to be injected subcutaneously (SC) or intramuscularly (IM) in the deltoid region or in the anterolateral area of the thigh.
Varilrix should be administered subcutaneously in individuals with bleeding disorders (e.g. thrombocytopenia or any coagulation disorder).
For instructions on reconstitution of the medicinal product before administration, see section 6.6.
Overdose
Cases of accidental administration of more than the recommended dose of Varilrix have been reported. Amongst these cases, the following adverse events were reported: lethargy and convulsions. In the other cases reported as overdose there were no associated adverse events.
Shelf life
2 years.
After reconstitution, it is recommended that the vaccine be injected as soon as possible.
However, it has been demonstrated that the reconstituted vaccine may be kept for up to 90 minutes at room temperature (25°C) and up to 8 hours in the refrigerator (2°C to 8°C). If not used within the recommended in-use storage timeframes and conditions, the reconstituted vaccine must be discarded.
Special precautions for storage
Store and transport refrigerated (2°C to 8°C).
Store in the original package in order to protect from light.
For storage conditions after reconstitution of the medicinal product, see section 6.3.
Nature and contents of container
Powder in a single-dose glass vial (type I glass) with a stopper (bromobutyl rubber).
0.5 ml of solvent in a pre-filled syringe (type I glass) with plunger stopper (bromobutyl rubber), with or without separate needles in the following pack sizes:
- with 1 separate needle: pack sizes of 1 or 10.
- with 2 separate needles: pack sizes of 1 or 10.
- without needle: pack sizes of 1 or 10.
0.5 ml of solvent in an ampoule (type I glass).
Pack size of 10.
Not all pack sizes may be marketed.
Special precautions for disposal and other handling
The solvent and the reconstituted vaccine should be inspected visually for any foreign particulate matter and/or abnormal physical appearance before administration. In the event of either being observed, do not administer the vaccine.
The vaccine must be reconstituted by adding the entire contents of the pre-filled syringe or ampoule of solvent to the vial containing the powder.
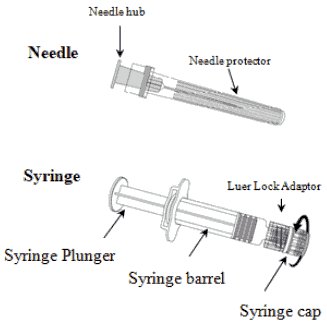
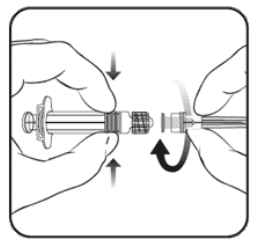
To attach the needle to the syringe, carefully read the instructions given with pictures 1 and 2. However, the syringe provided with Varilrix might be slightly different (without screw head) than the syringe illustrated. In that case, the needle should be attached without screwing.
Picture 1:
Picture 2:
Always hold the syringe by the barrel, not by the syringe plunger or the Luer Lock Adaptor (LLA), and maintain the needle in the axis of the syringe (as illustrated in picture 2). Failure to do this may cause the LLA to become distorted and leak.
During assembly of the syringe, if the LLA comes off, a new vaccine dose (new syringe and vial) should be used.
1. Unscrew the syringe cap by twisting it anticlockwise (as illustrated in picture 1).
Whether the LLA is rotating or not, please follow the below steps:
2. Attach the needle to the syringe by gently connecting the needle hub into the LLA and rotate a quarter turn clockwise until you feel it lock (as illustrated in picture 2).
3. Remove the needle protector, which may be stiff.
4. Add the solvent to the powder. The mixture should be well shaken until the powder is completely dissolved in the solvent.
The colour of the reconstituted vaccine may vary from clear peach to pink due to minor variations of its pH. This is normal and does not impair the performance of the vaccine. In the event of other variation being observed, do not administer the vaccine.
5. Withdraw the entire contents of the vial.
6. A new needle should be used to administer the vaccine. Unscrew the needle from the syringe and attach the injection needle by repeating step 2 above.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.