VARUBY Film-coated tablet Ref.[7569] Active ingredients: Rolapitant
Source: European Medicines Agency (EU) Revision Year: 2020 Publisher: TESARO Bio Netherlands B.V., Joop Geesinkweg 901, 1114 AB Amsterdam-Duivendrecht, Netherlands
Pharmacodynamic properties
Pharmacotherapeutic group: Antiemetics and antinauseants, other antiemetics
ATC code: A04AD14
Mechanism of action
Rolapitant is a selective antagonist of human substance P/neurokinin 1 (NK1) receptors.
Clinical efficacy and safety
Cisplatin-Based Highly Emetogenic Chemotherapy (HEC)
Study 1 and Study 2 (HEC)
In two multicentre, randomised, double-blind, parallel group, controlled clinical studies (Study 1 and Study 2), the rolapitant regimen (180 mg rolapitant, 10 mcg/kg intravenous granisetron and 20 mg oral dexamethasone) was compared with control therapy (placebo, 10 mcg/kg intravenous granisetron and 20 mg oral dexamethasone) on Day 1 in patients receiving a chemotherapy regimen that included cisplatin ≥60 mg/m². On Day 2 to 4, patients received 8 mg twice daily of oral dexamethasone. Study medicinal products were administered prior to chemotherapy on Day 1 at the following intervals: rolapitant (1 to 2 hours prior); granisetron and dexamethasone (30 minutes prior).
A total of 1087 patients were randomised to either the rolapitant regimen (N=544) or control therapy (N=543) across Study 1 and Study 2; 1070 patients were included in the evaluation of efficacy; 37% were women and 63% were men. Of the 1070 patients, 26% were greater than 65 years of age and 3% were greater than 75 years of age.
The primary endpoint in both studies was complete response (defined as no emetic episodes and no rescue medicinal product) in the delayed phase (>24 to 120 hours) of chemotherapy-induced nausea and vomiting. The following additional pre-specified endpoints were also evaluated: complete response in the acute phase (0 to 24 hours) and overall phase (0 to 120 hours); no emesis in each CINV phase, no significant nausea in each CINV phase, and time to first emesis or use of rescue medicinal product.
The results were evaluated for each individual study and for the two studies combined. Individual results from Studies 1 and 2 as well as a summary of the key results from the combined analysis are shown in Table 1 below.
Table 1. Proportion of patients receiving cisplatin chemotherapy responding by treatment group and phase (Studies 1 and 2 – HEC Individual Results):
| Efficacy Endpointsa | HEC Study 1 | HEC Study 2 | Study 1 and 2 Combined | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Rolapitant (N=264) Rate (%) | Control (N=262) Rate (%) | P-Valueb | Rolapitant (N=271) Rate (%) | Control (N=273) Rate (%) | P-Valueb | Rolapitant (N=535) Rate (%) | Control (N=535) Rate (%) | P-Valuec | |
| Complete Response | |||||||||
| Delayed | 72.7 | 58.4 | <0.001 | 70.1 | 61.9 | 0.043 | 71.4 | 60.2 | <0.001 |
| Acute | 83.7 | 73.7 | 0.005 | 83.4 | 79.5 | N.S. | 83.6 | 76.6 | 0.004 |
| Overall | 70.1 | 56.5 | 0.001 | 67.5 | 60.4 | N.S. | 68.8 | 58.5 | <0.001 |
| No Emesis | |||||||||
| Acute | 86.4 | 76.0 | 0.002 | 85.6 | 81.7 | N.S. | 86.0 | 78.9 | 0.002 |
| Delayed | 78.0 | 61.8 | <0.001 | 73.1 | 65.2 | 0.046* | 75.5 | 63.6 | <0.001 |
| Overall | 75.4 | 59.2 | <0.001 | 70.8 | 64.1 | N.S. | 73.1 | 61.7 | <0.001 |
| No Significant Nausea | |||||||||
| Acute | 86.4 | 79.4 | 0.035 | 90.0 | 85.7 | N.S. | 88.2 | 82.6 | 0.009 |
| Delayed | 73.5 | 64.9 | 0.034 | 74.5 | 68.9 | N.S. | 74.0 | 66.9 | 0.011 |
| Overall | 71.6 | 63.0 | 0.037 | 72.7 | 67.8 | N.S. | 72.1 | 65.4 | 0.017 |
a Primary endpoint was complete response in the delayed phase. Delayed phase: >24 to 120 hours post-cisplatin treatment; Acute phase: 0 to 24 hours post-cisplatin treatment; Overall phase: 0 to 120 hours post-cisplatin treatment
b Unadjusted P-values are obtained from Cochran-Mantel Haenszel test, stratified for sex.
c Unadjusted P-values are obtained from Cochran-Mantel-Haenszel test, stratified by study and sex.
N.S.=Not significant (p>0.05)
* Not significant after applying pre-specified multiplicity adjustment.
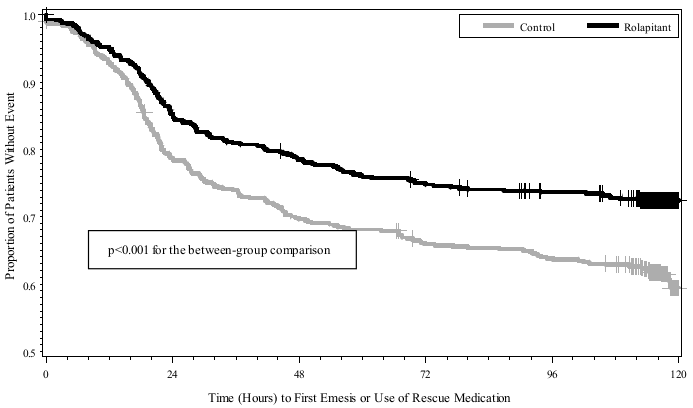
The estimated time to first emesis in the combined analysis is depicted by the Kaplan-Meier plot in Figure 1.
Figure 1. Kaplan-Meier Plot of Proportions of Patients without Emesis or Use of Rescue Medication (Study 1 and Study 2 Combined – HEC):
Moderately Emetogenic Chemotherapy and Combinations of Anthracycline and Cyclophosphamide Chemotherapy
Study 3 (MEC)
In Study 3, a multicentre, randomised, double-blind, parallel group, controlled clinical study in moderately emetogenic chemotherapy, the rolapitant regimen (180 mg rolapitant, 2 mg oral granisetron and 20 mg oral dexamethasone) was compared with control therapy (placebo, 2 mg oral granisetron and 20 mg oral dexamethasone) on Day 1 in patients receiving a moderately emetogenic chemotherapy regimen that included 53% of patients receiving a combination of anthracycline and cyclophosphamide (AC). On Day 2 to 3, patients received 2 mg once daily of oral granisetron. Study medicinal products were administered prior to chemotherapy on Day 1 at the following intervals: rolapitant (1 to 2 hours prior); granisetron and dexamethasone (30 minutes prior). At the time the study was designed, AC containing chemotherapy regimens were considered to be moderately emetogenic. Recent guidance has updated these regimens to highly emetogenic. The percentage of patients who received carboplatin in Cycle 1 was 30%.
A total of 1369 patients were randomised to either the rolapitant regimen (N=684) or control therapy (N=685). A total of 1332 patients were included in the evaluation of efficacy, 80% were women and 20% were men. Of these 1332 patients, 28% were greater than 65 years of age and 6% were greater than 75 years of age. Of these 1332 patients, 629 received non-AC chemotherapy.
The primary endpoint was complete response (defined as no emetic episodes and no rescue medicinal product) in the delayed phase (>24 to 120 hours) of chemotherapy-induced nausea and vomiting. The following additional pre-specified endpoints were also evaluated: complete response in the acute phase (0 to 24 hours) and overall phase (0 to 120 hours); no emesis in each CINV phase, no significant nausea in each CINV phase and time to first emesis or use of rescue medicinal product.
A summary of the study results from the MEC Study (Study 3) is shown in Table 2 below. A summary of the results from the non-AC and AC subsets are provided in Table 3.
Table 2. Proportion of Patients Receiving Moderately Emetogenic Chemotherapy Responding by Treatment Group and Phase:
| Study 3–MEC | |||
|---|---|---|---|
| Efficacy Endpointsa | Rolapitant (N=666) Rate (%) | Control (N=666) Rate (%) | P-Valueb |
| Complete Response | |||
| Delayed | 71.3 | 61.6 | <0.001 |
| Acute | 83.5 | 80.3 | N.S. |
| Overall | 68.6 | 57.8 | <0.001* |
| No Emesis | |||
| Acute | 87.8 | 84.5 | N.S. |
| Delayed | 80.5 | 69.8 | <0.001* |
| Overall | 78.7 | 65.3 | <0.001* |
| No Significant Nausea (maximum VAS <25 on 0-100 scale) | |||
| Acute | 82.1 | 84.7 | N.S. |
| Delayed | 72.7 | 69.4 | N.S. |
| Overall | 70.6 | 66.5 | N.S. |
a Primary endpoint was complete response in the delayed phase. Acute phase: 0 to 24 hours after AC or non-AC regimen; Delayed phase: >24 to 120 hours after AC or non-AC regimen; Overall phase: 0 to 120 hours after AC or non-AC regimen
b Unadjusted P-values are obtained from Cochran-Mantel-Haenszel test, stratified by sex.
N.S.=Not significant (p>0.05)
* N.S. after pre-specified multiplicity adjustment.
Table 3. Proportion of Patients Receiving AC or non-AC Chemotherapy Achieving Complete Response:
| Complete Response | Rolapitant | Control | P-Valuea |
|---|---|---|---|
| Non-AC | N=322 | N=307 | |
| Delayed | 76.1 | 63.8 | <0.001 |
| Acute | 90.7 | 84.4 | 0.016 |
| Overall | 74.8 | 61.2 | <0.001 |
| Με AC | N=344 | N=359 | |
| Delayed | 66.9 | 59.6 | 0.047 |
| Acute | 76.7 | 76.9 | N.S. |
| Overall | 62.8 | 54.9 | 0.033 |
a Unadjusted P-values are obtained from Cochran-Mantel-Haenszel test.
N.S.=Not significant (p>0.05)
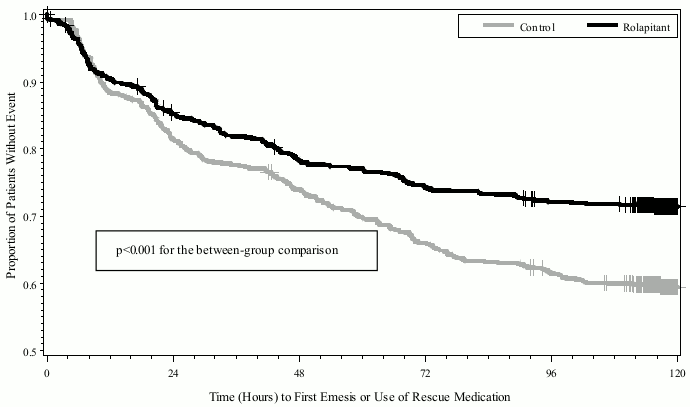
The estimated time to first emesis or use of rescue medicinal product in patients receiving a MEC regimen is depicted by the Kaplan-Meier plot in Figure 2.
Figure 2. Kaplan-Meier Plot of Proportions of Patients without Emesis or Use of Rescue Medication (Study 3-MEC):
The impact of nausea and vomiting on patients' daily lives was assessed using the Functional Living Index-Emesis (FLIE). The proportion of patients with no impact on daily life was higher in the Varuby group than in the control group (MEC: 73.2% vs. 67.4%; p=0.027).
Multiple-Cycle Extension
In each study, patients had the option of continuing into a multiple-cycle extension for up to 5 additional cycles of chemotherapy receiving the same treatment as assigned in cycle 1. At Day 6 to 8 following initiation of chemotherapy, patients were asked to recall whether they had any episode of vomiting or retching or nausea that interfered with normal daily life. Antiemetic activity of rolapitant was maintained throughout repeat cycles for those patients continuing in each of the multiple cycles.
Paediatric population
The European Medicines Agency has deferred the obligation to submit the results of studies with rolapitant in all subsets of the paediatric population in prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of highly emetogenic cisplatin-based cancer therapy and moderately emetogenic cancer therapy (see section 4.2 for information on paediatric use).
Pharmacokinetic properties
Rolapitant displays linear PK with exposures increased in a dose-proportional manner. Rolapitant is slowly eliminated with a mean terminal half-life of approximately 7 days. Rolapitant is eliminated mainly through the hepatobiliary route, with minor contributions from renal elimination. Rolapitant is metabolised by CYP3A4 to form a major active metabolite, M19. In vitro studies suggest that rolapitant is not an inhibitor of CYP2E1.
Absorption
Following a single dose administration of 180 mg rolapitant under fasting conditions to healthy subjects, rolapitant was measurable in plasma between 30 minutes and the peak plasma concentration (Cmax) for rolapitant which was reached in about 4 hours and mean Cmax was 968 ng/mL (CV:28).
Following multiple oral doses 9 to 45 mg once daily of rolapitant; accumulation of rolapitant was approximately 5-fold.
The systemic exposures (Cmax and AUC) to rolapitant increased in a dose-proportional manner when the dose of rolapitant increased from 4.5 mg to 180 mg. With an increase in dose by 4 times from the recommended clinical dose of 180 mg, the Cmax and AUC of rolapitant increased by 3.1 fold and 3.7 fold, respectively.
The absolute bioavailability of rolapitant is approximately 100%, indicating minimal first pass effect.
Concomitant administration of a high fat meal did not significantly affect the pharmacokinetics of rolapitant after administration of 180 mg rolapitant.
Distribution
Rolapitant was highly protein bound to human plasma (99.8%). The apparent volume of distribution (Vd/F) was 460 L in healthy subjects, indicating an extensive tissue distribution of rolapitant. In a population pharmacokinetic analysis of rolapitant, the Vd/F was 387 L in cancer patients.
Biotransformation
Rolapitant is metabolised by CYP3A4 to form a major active metabolite, M19 (C4-pyrrolidine-hydroxylated rolapitant). In a mass balance study, the metabolite M19 was the major circulating metabolite. The formation of M19 was significantly delayed with the median Tmax of 120 hours (range: 24-168 hours) and the mean half-life of M19 was 158 hours. The exposure ratio of M19 to rolapitant was approximately 50% in plasma.
Elimination
Following single oral doses (4.5 to 180 mg) of rolapitant, the mean terminal half-life (t½) of rolapitant ranged from 169 to 183 hours (approximately 7 days) and was independent of dose. In a population pharmacokinetic analysis the apparent total clearance (CL/F) of rolapitant was 0.96 L/hour in cancer patients.
Rolapitant is eliminated primarily through the hepatobiliary route. Following administration of a single oral 180-mg dose of [14C]-rolapitant, on average 14.2% (range 9% to 20%) and 73% (range 52% to 89%) of the dose was recovered in the urine and feces, respectively over 6 weeks. In pooled samples collected over 2 weeks, 8.3% of the dose was recovered in the urine primarily as metabolites and 37.8% of the dose was recovered in the feces primarily as unchanged rolapitant. Unchanged rolapitant or M19 were not found in pooled urine sample. Drug metabolising enzymes (and drug transporters) other than CYP3A4 involved in rolapitant hepatobiliary elimination remain to be elucidated.
Pharmacokinetics in special populations
Age, Sex and Race/Ethnicity
Population pharmacokinetic analyses indicated that age, sex and race had no significant impact on the pharmacokinetics of Varuby. There are limited data in patients aged 75 years and older.
Hepatic Impairment
Following administration of a single dose of 180 mg rolapitant to patients with mild hepatic impairment (Child-Pugh Class A), the pharmacokinetics of rolapitant were comparable with those of healthy subjects. In patients with moderate hepatic impairment (Child-Pugh Class B), the mean Cmax was 25% lower while mean AUC of rolapitant was similar compared to those of healthy subjects. The median Tmax for M19 was delayed to 204 hours in patients with mild or moderate hepatic impairment compared to 168 hours in healthy subjects. The pharmacokinetics of Varuby was not studied in patients with severe hepatic impairment (Child-Pugh Class C).
Renal Impairment
In population pharmacokinetic analyses, creatinine clearance (CLcr) at baseline did not show a significant effect on rolapitant pharmacokinetics in cancer patients with mild (CLcr: 60 to 90 mL/min) or moderate (CLcr: 30 to 60 mL/min) renal impairment compared to cancer patients with normal kidney function. Information is insufficient for the effect of severe renal impairment. The pharmacokinetics of Varuby was not studied in patients with end-stage renal disease requiring haemodialysis.
Relationship between concentration and effect
NK1 Receptor Occupancy
A human Positron Emission Tomography (PET) study with rolapitant demonstrated that rolapitant crosses the blood brain barrier and occupies brain NK1 receptors. A dose-dependent increase in mean NK1 receptor occupancy was observed in the dose range from 4.5 mg to 180 mg of rolapitant. At rolapitant plasma concentrations of >15 ng/mL and 348 ng/mL, the NK1 receptor occupancies in the cortical regions were approximately >50% and 90% respectively. At the 180 mg dose of rolapitant, the mean NK1 receptor occupancy in the cortical regions was greater than 90% for at least 120 hours.
Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, genotoxicity, teratogenic potential, and carcinogenic potential.
The mechanism of the significant difference of half-lives observed between the rat and monkey (6-8 h) and human (7 days) is not elucidated.
In rodents, rolapitant was tested in repeated dose oral toxicity studies up to 26 weeks in duration, and the liver, thyroid, kidneys, epididymis and uterus were identified as target organs. In a three-month rat study, clonic convulsions were observed in a single animal at 125 mg/kg/day (approximately 6 times the recommended human dose on a body surface area basis). In the one-month monkey study, convulsions were observed at 60 mg/kg/day (approximately 5.8 times the recommended human dose on a body surface area basis). The relevance of convulsions for humans is unknown.
In a fertility and early embryonic development study in female rats, rolapitant hydrochloride at an oral dose equivalent to 9 mg/kg per day free base (approximately 0.5 times the recommended human dose on a body surface area basis) caused a transient decrease in maternal body weight gain and increases in the incidence of pre- and post-implantation loss. At a dose equivalent to 4.5 mg/kg per day free base (approximately 0.2 times the recommended human dose on a body surface area basis), there were decreases in the number of corpora lutea and implantation sites.
In a pre- and post-natal development rat study, maternal toxicity was evident based on mortality/moribund condition, decreased body weight and food consumption, total litter loss, prolonged parturition, decreased length of gestation, and increased number of unaccounted for implantation sites at a dose equivalent to 22.5 mg/kg per day free base (approximately 1.2 times the recommended human dose on a body surface area basis). Effects on offspring at this dose included decreased postnatal survival, and decreased body weights and body weight gain, and may be related to the maternal toxicity observed. At a maternal dose equivalent to 9 mg/kg per day rolapitant free base (approximately 0.5 times the recommended human dose on a body surface area basis), there was a decrease in memory in female pups in a maze test and a decrease in pup body weight.
Based on the environmental risk assessment, rolapitant is considered as very persistent, bioaccumulative and not readily biodegradable.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.