VERQUVO Film-coated tablet Ref.[27620] Active ingredients: Vericiguat
Source: European Medicines Agency (EU) Revision Year: 2021 Publisher: Bayer AG, 51368 Leverkusen, Germany
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Cardiac therapy, other vasodilators used in cardiac diseases
ATC code: C01DX22
Mechanism of action
Vericiguat is a stimulator of soluble guanylate cyclase (sGC). Heart failure is associated with impaired synthesis of nitric oxide (NO) and decreased activity of its receptor, sGC. Deficiency in sGC-derived cyclic guanosine monophosphate (cGMP) contributes to myocardial and vascular dysfunction. Vericiguat restores the relative deficiency in the NO-sGC-cGMP signalling pathway by directly stimulating sGC, independently of and synergistically with NO, to augment the levels of intracellular cGMP, which may improve both myocardial and vascular function.
Pharmacodynamic effects
The pharmacodynamic effects of vericiguat are consistent with the mode of action of a sGC stimulator resulting in smooth muscle relaxation and vasodilation.
In a 12-week placebo-controlled dose-finding study (SOCRATES-REDUCED) in patients with heart failure, vericiguat demonstrated a dose-dependent reduction in NT-proBNP, a biomarker in heart failure, compared to placebo when added to standard of care. In VICTORIA, the estimated reduction from baseline NT-proBNP at week 32 was greater in patients who received vericiguat compared with placebo (see clinical efficacy and safety).
Cardiac electrophysiology
In a dedicated QT study in patients with stable coronary artery disease, administration of 10 mg of vericiguat at steady state did not prolong the QT interval to a clinically relevant extent, i.e. the maximum mean prolongation of the QTcF interval did not exceed 6 ms (upper bound of the 90% CI <10 ms).
Clinical efficacy and safety
The safety and efficacy of vericiguat were evaluated in a randomised, parallel-group, placebocontrolled, double-blind, event-driven, multi-centre trial (VICTORIA) comparing vericiguat and placebo in 5,050 adult patients with symptomatic chronic heart failure (NYHA class II–IV) and left ventricular ejection fraction (LVEF) less than 45% following a worsening heart failure (HF) event. A worsening chronic HF event was defined as heart failure hospitalisation within 6 months before randomisation or use of outpatient IV diuretics for heart failure within 3 months before randomisation.
Patients were treated up to the target maintenance dose of vericiguat 10 mg once daily or matching placebo in combination with other HF therapies. Therapy was initiated at 2.5 mg vericiguat once daily and increased in approximately 2 week intervals to 5 mg once daily and then 10 mg once daily, as tolerated. After approximately 1 year, 89% of vericiguat-treated patients and 91% of placebo-treated patients received the 10 mg target dose in addition to other HF therapies.
The primary endpoint was the time to first event of the composite of cardiovascular (CV) death or hospitalisation for HF. The median follow-up for the primary endpoint was 11 months. Patients on vericiguat were treated for a mean duration of 1 year and up to 2.6 years.
The mean age of the studied population was 67 years, a total of 1,596 (63%) patients treated with vericiguat were 65 years and older, and 783 (31%) patients treated with vericiguat were 75 years and older. At randomisation, 58.9% of patients were NYHA Class II, 39.7% were NYHA Class III, and 1.3% were NYHA Class IV. The mean LVEF was 28.9%, approximately half of all patients had an LVEF <30%, and 14.3% of patients had an LVEF between 40% and 45%. The most frequently reported medical history conditions other than HF included hypertension (79%), coronary artery disease (58%), hyperlipidaemia (57%), diabetes mellitus (47%), atrial fibrillation (45%), and myocardial infarction (42%). At randomisation, the mean eGFR was 62 mL/min/1.73 m² (88% of patients >30 mL/min/1.73 m²; 10% of patients ≤30 mL/min/1.73 m²). 67% of the patients in VICTORIA were enrolled within 3 months of a HF hospitalisation; 17% were enrolled within 3 to 6 months of HF hospitalisation and 16% were enrolled within 3 months of outpatient treatment with IV diuretics. The median NT-proBNP level was 2,816 pg/mL at randomisation.
At baseline, more than 99% of patients were treated with other HF therapies which included beta blockers (93%), angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARB) (73%), mineralocorticoid receptor antagonists (MRA) (70%), a combination of an angiotensin receptor and neprilysin inhibitor (ARNI) (15%), ivabradine (6%), implantable cardiac defibrillators (28%), and biventricular pacemakers (15%). 91% of patients were treated with 2 or more HF medicinal products (beta blocker, any renin-angiotensin system [RAS] inhibitor, or MRA) and 60% of patients were treated with all 3. 3% of patients were on a sodium glucose co-transporter 2 (SGLT2) inhibitor.
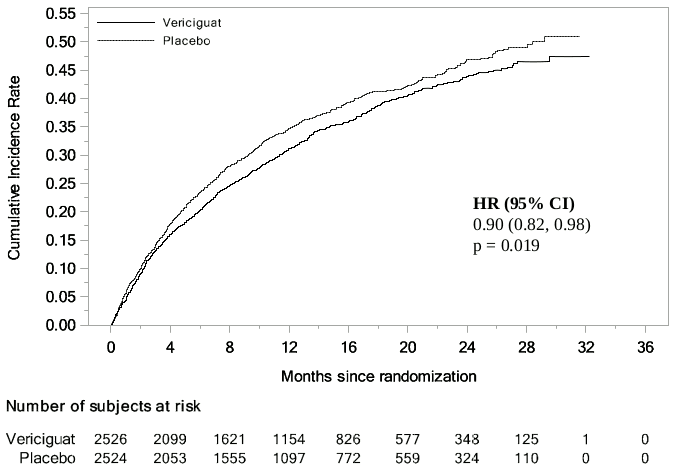
Vericiguat was superior to placebo in reducing the risk of CV death or HF hospitalisation based on a time-to-event analysis. Over the course of the study, the annualised absolute risk reduction (ARR) was 4.2% with vericiguat compared with placebo. Therefore, 24 patients would need to be treated over an average of 1 year to prevent 1 primary endpoint event. The treatment effect reflected a reduction in the risk of CV death, HF hospitalisation, all-cause mortality or HF hospitalisation and total number of HF hospitalisation (see table 2 and figure 1).
Table 2. Treatment effect for the primary composite endpoint, its components, and the secondary endpoints:
| Vericiguat N=2,526 | Placebo N=2,524 | Treatment comparison | |
|---|---|---|---|
| n (%) [Annual %1] | n (%) [Annual %1] | Hazard Ratio (95% CI)2 [Annualised ARR %]4 | |
| Primary endpoint | |||
| Composite of CV death or HF hospitalisation5 | 897 (35.5) [33.6] | 972 (38.5) [37.8] | 0.90 (0.82, 0.98) p = 0.0193 [4.2] |
| CV death | 206 (8.2) | 225 (8.9) | |
| HF hospitalisation | 691 (27.4) | 747 (29.6) | |
| Secondary endpoints | |||
| CV death | 414 (16.4) [12.9] | 441 (17.5) [13.9] | 0.93 (0.81, 1.06) |
| HF hospitalisation | 691 (27.4) [25.9] | 747 (29.6) [29.1] | 0.90 (0.81, 1.00) |
| Composite of all-cause mortality or HF hospitalisation5 | 957 (37.9) [35.9] | 1,032 (40.9) [40.1] | 0.90 (0.83, 0.98) |
| Total number of HF hospitalisations (first and recurrent) | 1,223 [38.3] | 1,336 [42.4] | 0.91 (0.84, 0.99)6 |
1 Total patients with an event per 100 patient years at risk.
2 Hazard ratio (vericiguat over placebo) and confidence interval from a Cox proportional hazards model.
3 From the log-rank test. p-value applies to HR only and not annualised ARR.
4 Annualised absolute risk reduction, calculated as difference (placebo-vericiguat) in annual %.
5 For patients with multiple events, only the first event contributing to the composite endpoint is counted.
6 Hazard ratio (vericiguat over placebo) and confidence interval from an Andersen-Gill model.
N=Number of patients in Intent-to-treat (ITT) population; n=Number of patients with an event.
Figure 1. Kaplan-Meier curve for the primary composite endpoint: time to first occurrence of CV death or HF hospitalisation:
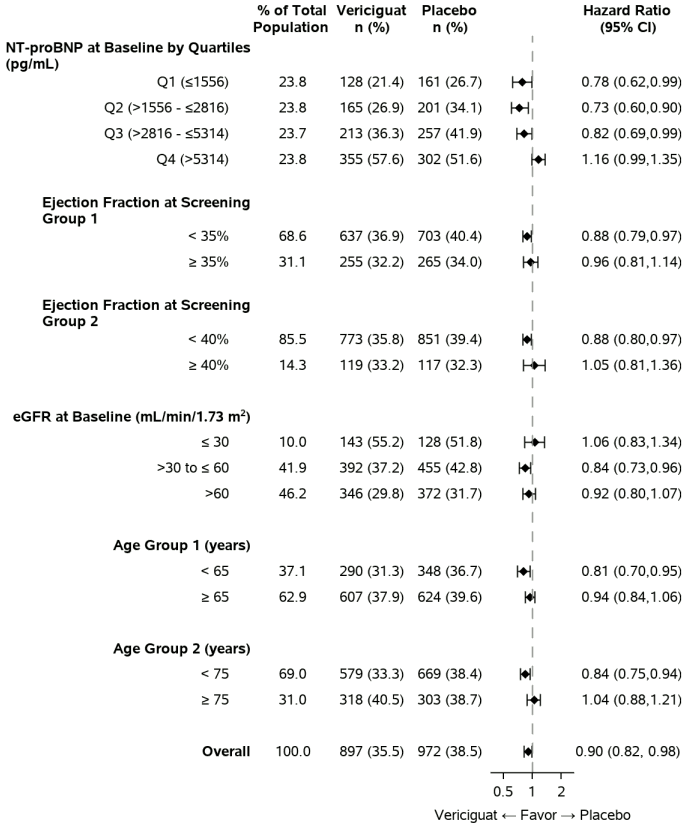
A wide range of demographic characteristics, baseline disease characteristics and baseline concomitant medicinal products were examined for their influence on outcomes. The results of the primary composite endpoint were generally consistent across subgroups. Results of select pre-specified subgroup analyses are shown in figure 2.
Figure 2. Primary composite endpoint (time to first occurrence of CV death or HF hospitalisation) - select subgroups of the pre-specified analyses:
Patients with very high NT-proBNP may not be fully stabilised and require further optimisation of volume status and diuretic therapy (see sections 4.1 and 4.2).
Paediatric population
The European Medicines Agency has deferred the obligation to submit the results of studies with Verquvo in one or more subsets of the paediatric population in the treatment of left ventricular failure (see section 4.2 for information on paediatric use).
5.2. Pharmacokinetic properties
General introduction
Vericiguat shows time-independent pharmacokinetics with low to moderate variability when administered with food. Pharmacokinetics are dose proportional in healthy volunteers and slightly less than dose proportional in heart failure patients. Vericiguat accumulates in plasma up to 155-171% and reaches pharmacokinetic steady state after approximately 6 days. The mean steady-state population pharmacokinetic parameters of vericiguat in heart failure patients are summarised in table 3. Steadystate exposure is estimated to be about 20% higher in heart failure patients when compared to healthy volunteers.
Table 3. Population pharmacokinetic model based steady-state geometric mean (CV%) plasma pharmacokinetic (PK) parameters of 2.5 mg, 5 mg, or 10 mg vericiguat in heart failure patients (N=2,321):
| PK Parameters | 2.5 mg | 5 mg | 10 mg |
|---|---|---|---|
| Cmax (µg/L) | 120 (29.0) | 201 (29.0) | 350 (29.0) |
| AUC (µg•h/L) | 2,300 (33.9) | 3,850 (33.9) | 6,680 (33.9) |
Absorption
The absolute bioavailability of vericiguat is high (93%) when taken with food. Bioavailability (AUC) and peak plasma levels (Cmax) of vericiguat administered orally as a crushed tablet in water are comparable to that of a whole tablet (see section 4.2).
Effect of food
Administration of vericiguat with a high-fat, high-calorie meal increases Tmax from about 1 hour (fasted) to about 4 hours (fed), reduces PK variability, and increases vericiguat exposure by 19% (AUC) and 9% (Cmax) for the 5 mg tablet and by 44% (AUC) and 41% (Cmax) for the 10 mg tablet as compared with the fasted state. Similar results were obtained when vericiguat was administered with a low-fat, high-carbohydrate meal. Therefore, Verquvo should be taken with food (see section 4.2).
Distribution
The mean steady-state volume of distribution of vericiguat in healthy subjects is approximately 44 L. Plasma protein binding of vericiguat is about 98%, with serum albumin being the main binding component. Plasma protein binding of vericiguat is not altered by renal or hepatic impairment.
Biotransformation
Glucuronidation is the major biotransformation pathway of vericiguat to form an N-glucuronide, which is pharmacologically inactive and the major drug-related component in plasma, accounting for 72% of the total drug-related AUC, with the parent vericiguat accounting for 28% of the total drugrelated AUC. N-glucuronidation is catalysed predominantly by UGT1A9, as well as UGT1A1. CYP-mediated metabolism is a minor clearance pathway (<5%).
The potential effect of UGT-related genetic polymorphism has not been investigated given the low-tomoderate inter-individual variability of vericiguat (see table 3). Titration of vericiguat mitigates the clinical impact of potential changes in exposure (see section 4.2).
Elimination
Vericiguat is a low-clearance drug (1.6 L/h in healthy subjects). The half-life is about 20 hours in healthy subjects and 30 hours in heart failure patients. Following oral administration of [14C]-vericiguat to healthy subjects, approximately 53% of the dose was excreted in urine (primarily as the N-glucuronide), and 45% of the dose was excreted in faeces (primarily as vericiguat, likely due to excretion of the N-glucuronide into bile followed by hydrolysis back to vericiguat by intestinal microflora).
Special populations
Renal impairment
In patients with heart failure with mild, moderate, and severe renal impairment not requiring dialysis, the mean exposure (AUC) of vericiguat was increased by 5%, 13%, and 20% respectively, compared to patients with normal renal function. These differences in exposure are not considered clinically relevant. The pharmacokinetics of vericiguat have not been studied in patients with eGFR <15 mL/min/1.73 m² at treatment initiation or on dialysis (see sections 4.2 and 4.4).
In a dedicated clinical pharmacology study, otherwise healthy participants with mild, moderate, and severe renal impairment, had 8%, 73%, and 143% respectively, higher mean vericiguat exposure (unbound AUC normalised for body weight) after a single dose compared to healthy controls. The apparent discrepancy of the effect of renal impairment on vericiguat exposure between the dedicated clinical pharmacology study and the analysis in patients with heart failure may be attributed to differences in study design and size.
Hepatic impairment
No relevant increase in exposure (unbound AUC) was observed for subjects with mild hepatic impairment (Child-Pugh A) with mean exposure to vericiguat 21% higher compared to healthy subjects with normal hepatic function. In subjects with moderate hepatic impairment (Child-Pugh B), mean exposure to vericiguat was approximately 47% higher compared to their healthy subjects with normal hepatic function. The pharmacokinetics of vericiguat have not been studied in patients with severe hepatic impairment (Child-Pugh C) (see sections 4.2 and 4.4).
Effects of age, body weight, gender, ethnicity, race and baseline NT-proBNP
Based on an integrated population pharmacokinetic analysis of vericiguat in patients with heart failure, age (23-98 years), body weight, gender, ethnicity, race and baseline NT-proBNP do not have a clinically meaningful effect on the pharmacokinetics of vericiguat (see section 5.1).
Paediatric population
No studies with vericiguat have been performed yet in paediatric patients.
In vitro assessment of medicinal product interactions
Vericiguat is a substrate for UGT1A9, as well as UGT1A1 (see section 4.5). In vitro studies indicate that vericiguat and its N-glucuronide are neither inhibitors of major CYP isoforms (CYP1A2, 2B6, 2C8, 2C9, 2C19, 2D6 and 3A4) or UGT isoforms (UGT1A1, 1A4, 1A6, 1A9, 2B4, and 2B7), nor inducers of CYP1A2, 2B6 and 3A4, at clinically relevant concentrations.
Vericiguat is a substrate of P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP) transporters and is not a substrate of organic cation transporter (OCT1) or organic anion transporting polypeptides (OATP1B1, OATP1B3). Vericiguat and its N-glucuronide are not inhibitors of drug transporters, including P-gp, BCRP, BSEP, OATP1B1/1B3, OAT1, OAT3, OCT1, OCT2, MATE1, and MATE2K, at clinically relevant concentrations.
Overall, these data indicate that the administration of vericiguat is unlikely to affect the pharmacokinetics of concurrently administered medicinal products that are substrates of these enzymes or transporters.
5.3. Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity, carcinogenic potential, and male and female fertility.
In repeat-dose toxicity studies, the toxicological profile was characterised by effects secondary to exaggerated pharmacodynamics. Secondary to smooth muscle relaxation haemodynamic and gastrointestinal effects were noted in all species investigated. In adolescent rapidly-growing rats, reversible bone effects consisting of hypertrophy of growth plate and hyperostosis and remodelling of metaphyseal and diaphyseal bone were seen. These effects were not observed after chronic administration of vericiguat to adult rats and almost full-grown dogs.
A study in pregnant rats showed that vericiguat is transferred to the foetus through the placenta. Developmental toxicity studies in rats with vericiguat administered orally during organogenesis showed no developmental toxicity up to at least 21 times the human exposure (based on unbound AUC) at the maximum recommended human dose (MRHD) of 10 mg. In rabbits, late abortions and resorptions were observed, at maternally toxic doses at ≥6 times the human exposure at the MRHD. In a pre-/postnatal toxicity study in rats, at maternal toxic doses decreased pup body weight gain resulting in a slight delay in incisor eruption and a slight delay in vaginal opening was observed at approximately ≥21 times the human exposure at the MRHD. An increased incidence of stillbirths and decreased pup survival and a delay in balano-preputial separation were observed at 49 times the human exposure at the MRHD.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.