VESOMNI Modified release tablets Ref.[10456] Active ingredients: Solifenacin Tamsulosin
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2019 Publisher: Astellas Pharma Ltd., SPACE, 68 Chertsey road, Woking, Surrey, GU21 5BJ, UK
4.3. Contraindications
- Patients with hypersensitivity to the active substance(s) or to any of the excipients listed in section 6.1,
- Patients undergoing haemodialysis (see section 5.2),
- Patients with severe hepatic impairment (see section 5.2),
- Patients with severe renal impairment who are also treated with a strong cytochrome P450 (CYP) 3A4 inhibitor, e.g., ketoconazole (see section 4.5),
- Patients with moderate hepatic impairment who are also treated with a strong CYP3A4 inhibitor, e.g., ketoconazole (see section 4.5),
- Patients with severe gastrointestinal conditions (including toxic megacolon), myasthenia gravis or narrow-angle glaucoma and patients at risk for these conditions,
- Patients with a history of orthostatic hypotension.
4.4. Special warnings and precautions for use
Vesomni should be used with caution in patients with:
- severe renal impairment,
- risk of urinary retention,
- gastrointestinal obstructive disorders,
- risk of decreased gastrointestinal motility,
- hiatus hernia/gastroesophageal reflux and/or who are concurrently taking medicinal products (such as bisphosphonates) that can cause or exacerbate oesophagitis,
- autonomic neuropathy.
The patient should be examined in order to exclude the presence of other conditions, which can cause similar symptoms to benign prostatic hyperplasia.
Other causes of frequent urination (heart failure or renal disease) should be assessed before treatment with Vesomni is initiated. If a urinary tract infection is present, appropriate antibacterial therapy should be started.
QT prolongation and Torsade de Pointes have been observed in patients with risk factors, such as pre-existing long QT syndrome and hypokalaemia, who are treated with solifenacin succinate.
Angioedema with airway obstruction has been reported in some patients on solifenacin succinate and tamsulosin. If angioedema occurs, Vesomni should be discontinued and not restarted. Appropriate therapy and/or measures should be taken.
Anaphylactic reaction has been reported in some patients treated with solifenacin succinate. In patients who develop anaphylactic reactions, Vesomni should be discontinued and appropriate therapy and/or measures should be taken.
As with other alpha1-adrenoceptor antagonists, a reduction in blood pressure can occur in individual cases during treatment with tamsulosin, as a result of which, rarely, syncope can occur. Patients starting treatment with Vesomni should be cautioned to sit or lie down at the first signs of orthostatic hypotension (dizziness, weakness) until the symptoms have disappeared.
The ‘Intraoperative Floppy Iris Syndrome’ (IFIS, a variant of small pupil syndrome) has been observed during cataract and glaucoma surgery in some patients on or previously treated with tamsulosin hydrochloride. IFIS may increase the risk of eye complications during and after the operation. Therefore, the initiation of therapy with Vesomni in patients for whom cataract or glaucoma surgery is scheduled is not recommended. Discontinuing treatment with Vesomni 1-2 weeks prior to cataract or glaucoma surgery is anecdotally considered helpful, but the benefit of treatment discontinuation has not been established. During pre-operative assessment, surgeons and ophthalmic teams should consider whether patients scheduled for cataract or glaucoma surgery are being or have been treated with Vesomni in order to ensure that appropriate measures will be in place to manage IFIS during surgery.
Vesomni should be used with caution in combination with moderate and strong inhibitors of CYP3A4 (see section 4.5) and it should not be used in combination with strong inhibitors of CYP3A4, e.g., ketoconazole, in patients who are of the CYP2D6 poor metaboliser phenotype or who are using strong inhibitors of CYP2D6, e.g., paroxetine.
4.5. Interaction with other medicinal products and other forms of interaction
Concomitant medication with any medicinal products with anticholinergic properties may result in more pronounced therapeutic effects and undesirable effects. An interval of approximately one week should be allowed after stopping treatment with Vesomni, before commencing any anticholinergic therapy. The therapeutic effect of solifenacin may be reduced by concomitant administration of cholinergic receptor agonists.
Interactions with CYP3A4 and CYP2D6 inhibitors
Concomitant administration of solifenacin with ketoconazole (a strong inhibitor of CYP3A4) (200 mg/day) resulted in a 1.4- and 2.0-fold increase in Cmax and area under the curve (AUC) of solifenacin, while ketoconazole at a dose of 400 mg/day resulted in a 1.5- and 2.8-fold increase in Cmax and AUC of solifenacin.
Concomitant administration of tamsulosin with ketoconazole at a dose of 400 mg/day resulted in a 2.2- and 2.8-fold increase in Cmax and AUC of tamsulosin, respectively.
Since concomitant administration with strong inhibitors of CYP3A4, such as ketoconazole, ritonavir, nelfinavir and itraconazole may lead to increased exposure to both solifenacin and tamsulosin, Vesomni should be used with caution in combination with strong CYP3A4 inhibitors.
Vesomni should not be given together with strong CYP3A4 inhibitors in patients who are also CYP2D6 poor metaboliser phenotype or who are already using strong CYP2D6 inhibitors.
Concomitant administration of Vesomni with verapamil (a moderate CYP3A4 inhibitor) resulted in an approximately 2.2-fold increase in Cmax and AUC of tamsulosin and an approximately 1.6-fold increase in the Cmax and AUC of solifenacin. Vesomni should be used with caution in combination with moderate inhibitors of CYP3A4.
Concomitant administration of tamsulosin with the weak CYP3A4 inhibitor cimetidine (400 mg every 6 hours) resulted in a 1.44-fold increase in the AUC of tamsulosin, while Cmax was not significantly changed. Vesomni can be used with weak CYP3A4 inhibitors.
Concomitant administration of tamsulosin with the strong CYP2D6 inhibitor paroxetine (20 mg/day) resulted in an increase in Cmax and AUC of tamsulosin by 1.3- and 1.6-fold, respectively. Vesomni can be used with CYP2D6 inhibitors.
The effect of enzyme induction on the pharmacokinetics of solifenacin and tamsulosin has not been studied. Since solifenacin and tamsulosin are metabolised by CYP3A4, pharmacokinetic interactions are possible with CYP3A4 inducers (e.g., rifampicin) which may decrease the plasma concentration of solifenacin and tamsulosin.
Other Interactions
The following statements reflect the information available on the individual active substances.
Solifenacin
- Solifenacin can reduce the effect of medicinal products that stimulate the motility of the gastrointestinal tract, such as metoclopramide and cisapride.
- In vitro studies with solifenacin have demonstrated that at therapeutic concentrations, solifenacin does not inhibit CYP1A1/2, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1 or 3A4. Therefore, no interactions are expected between solifenacin and drugs metabolised by these CYP enzymes.
- Intake of solifenacin did not alter the pharmacokinetics of R-warfarin or S-warfarin or their effect on prothrombin time.
- Intake of solifenacin showed no effect on the pharmacokinetics of digoxin.
Tamsulosin
- Co-administration with other alpha1-adrenoceptor antagonists could lead to hypotensive effects.
- In vitro, the free fraction of tamsulosin in human plasma was not changed by diazepam, propranolol, trichlormethiazide, chlormadinone, amitriptyline, diclofenac, glibenclamide, simvastatin or warfarin. Tamsulosin does not change the free fraction of diazepam, propranolol, trichlormethiazide or chlormadinone. Diclofenac and warfarin, however, may increase the elimination rate of tamsulosin.
- Co-administration with furosemide causes a fall in plasma levels of tamsulosin, but as levels remain within the normal range, concurrent use is acceptable.
- In vitro studies with tamsulosin have demonstrated that at therapeutic concentrations, tamsulosin does not inhibit CYP1A2, 2C9, 2C19, 2D6, 2E1 or 3A4. Therefore, no interactions are expected between tamsulosin and drugs metabolised by these CYP enzymes.
- No interactions have been seen when tamsulosin was given concomitantly with atenolol, enalapril, or theophylline.
4.6. Fertility, pregnancy and lactation
Fertility
The effect of Vesomni on fertility has not been established. Animal studies with solifenacin or tamsulosin do not indicate harmful effects on fertility and early embryonic development (see section 5.3).
Ejaculation disorders have been observed in short and long term clinical studies with tamsulosin. Events of ejaculation disorder, retrograde ejaculation and ejaculation failure have been reported in the post authorization phase.
Pregnancy and lactation
Vesomni is not indicated for use in women.
4.7. Effects on ability to drive and use machines
No studies on the effects of Vesomni on the ability to drive or use machines have been performed. However, patients should be informed about the possible occurrence of dizziness, blurred vision, fatigue and uncommonly, somnolence, which may negatively affect the ability to drive or use machines (see section 4.8).
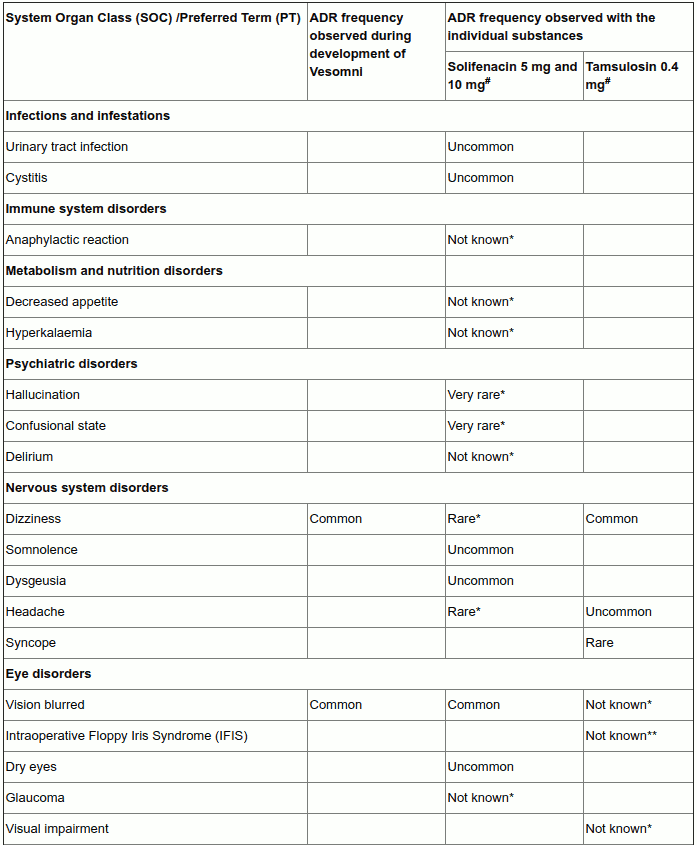
4.8. Undesirable effects
Summary of the safety profile
Vesomni may cause anticholinergic undesirable effects of, in general, mild to moderate severity. The most frequently reported adverse reactions during the clinical studies performed for the development of Vesomni were dry mouth (9.5%), followed by constipation (3.2%) and dyspepsia (including abdominal pain; 2.4%). Other common undesirable effects are dizziness (including vertigo; 1.4%), vision blurred (1.2%), fatigue (1.2%), and ejaculation disorder (including retrograde ejaculation; 1.5%). Acute urinary retention (0.3%, uncommon) is the most serious adverse drug reaction that has been observed during treatment with Vesomni in clinical studies.
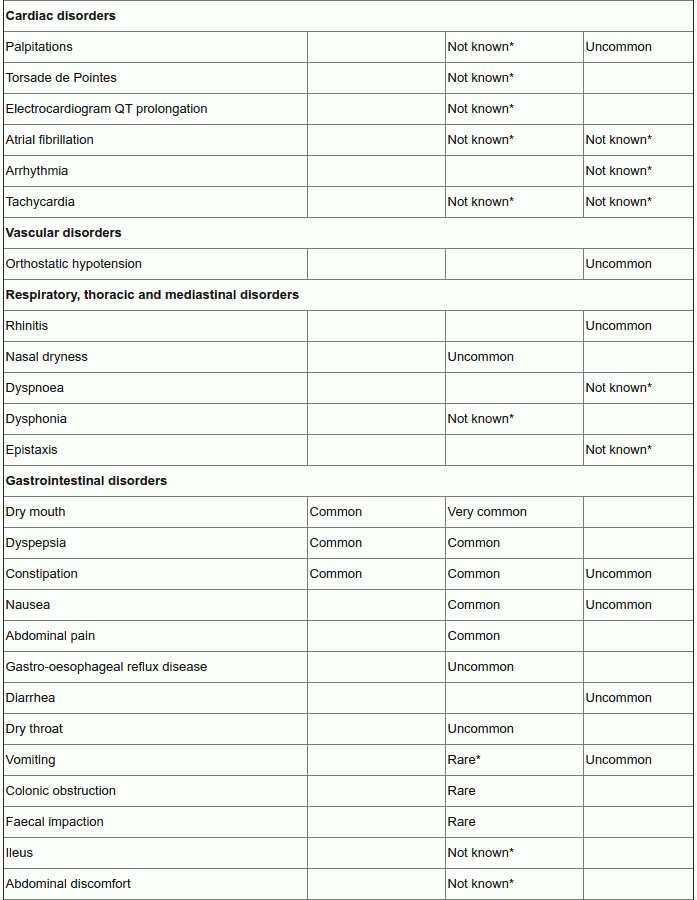
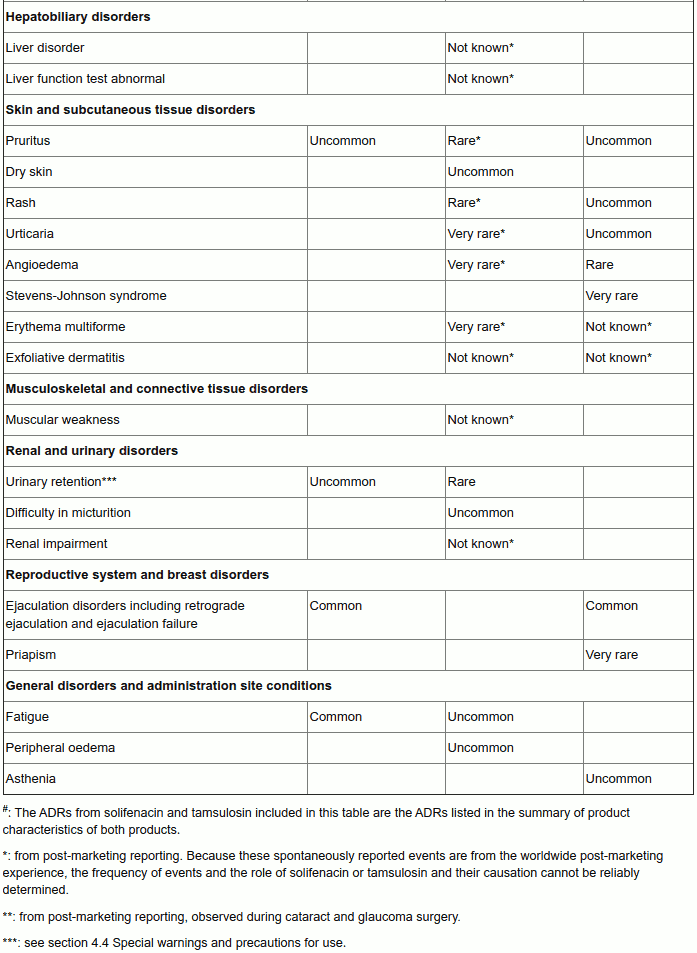
Tabulated list of adverse reactions
In the table below the ‘Vesomni frequency’ column reflects adverse drug reactions that have been observed during the double-blind clinical studies performed for the development of Vesomni (based on reports of treatment-related adverse events, which have been reported by at least two patients and occurred with a frequency higher than for placebo in the double-blind studies).
The columns ‘solifenacin frequency’ and ‘tamsulosin frequency’ reflect adverse drug reactions (ADRs) previously reported with one of the individual components (as presented in the Summary of Product Characteristics (SmPCs) of solifenacin 5 and 10 mg and tamsulosin 0.4 mg respectively) that may also occur when receiving Vesomni (some of these have not been observed during the clinical development program of Vesomni).
The frequency of adverse reactions is defined as follows: very common (≥1/10); common (≥1/100 to <1/10); uncommon (≥1/1,000 to <1/100); rare (≥1/10,000 to <1/1,000); very rare (<1/10,000), not known (cannot be estimated from the available data).
Long-term safety of Vesomni
The profile of undesirable effects seen with treatment up to 1 year was similar to that observed in the 12-week studies. The product is well-tolerated and no specific adverse reactions have been associated with long-term use.
Description of selected adverse reactions
For urinary retention see section 4.4 Special warnings and precautions for use.
Older people
The therapeutic indication of Vesomni, moderate to severe storage symptoms (urgency, increased micturition frequency) and voiding symptoms associated with BPH, is a disease affecting elderly men. The clinical development of Vesomni has been performed in patients 45 to 91 years of age, with an average age of 65 years. Adverse reactions in the elderly population were similar to the younger population.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or Apple App Store.
6.2. Incompatibilities
Not applicable.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.