VIADOX Solution for injection Ref.[50503] Active ingredients: Dexamethasone
Source: FDA, National Drug Code (US) Revision Year: 2022
Product description
Dexamethasone sodium phosphate injection, USP is a water-soluble inorganic ester of dexamethasone which produces a rapid response even when injected intramuscularly.
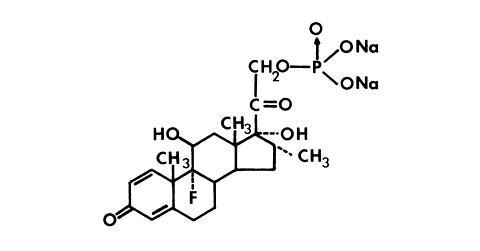
Dexamethasone sodium phosphate, a synthetic adrenocortical steroid, is a white or slightly yellow crystalline powder. It is freely soluble in water and is exceedingly hygroscopic. The molecular weight is 516.41. It is designated chemically as 9-fluoro-11β,17-dihydroxy-16α-methyl-21-(phosphonooxy)pregna-1,4-diene-3, 20-dione disodium salt.
The molecular formula is: C22H28FNa2O8P and the structural formula is:
Dexamethasone Sodium Phosphate Injection is a sterile solution of dexamethasone sodium phosphate for intravenous and intramuscular use. The 4 mg/mL strength may also be used for intra-articular, intralesional and soft tissue administration.
Each mL of Dexamethasone Sodium Phosphate Injection 4 mg/mL contains dexamethasone sodium phosphate, equivalent to 4 mg dexamethasone phosphate or 3.33 mg dexamethasone. Inactive ingredients per mL: 1 mg sodium sulfite anhydrous, 19.4 mg sodium citrate anhydrous and 10.42 mg (0.01 mL) benzyl alcohol (preservative) in Water for Injection.
Each mL of Dexamethasone Sodium Phosphate Injection 10 mg/mL contains dexamethasone sodium phosphate, equivalent to 10 mg dexamethasone phosphate or 8.33 mg dexamethasone. Inactive ingredients per mL: 1.5 mg sodium sulfite anhydrous, 16.5 mg sodium citrate anhydrous and 10.42 mg (0.01 mL) benzyl alcohol (preservative) in Water for Injection.
The pH of both concentrations is 7.0-8.5; sodium hydroxide and/or citric acid used, if needed, for pH adjustment. Sealed under nitrogen.
| How Supplied |
|---|
|
Dexamethasone Sodium Phosphate Injection, USP is available in the following package: 10 mg/mL - 1 mL vial Contents (NDC 70529-045-01): 1 - 1mL Dexamethasone Sodium Phosphate (10mg/mL) Contents (NDC 70529-045-02): 2 - 1mL Dexamethasone Sodium Phosphate (10mg/mL) Contents (NDC 70529-045-05): 5 - 1mL Dexamethasone Sodium Phosphate (10mg/mL) Assembled and Distributed by IT3 Medical, LLC, 190 E Stacy Road; STE 306-298 Allen, TX 75002-8734 |
Drugs
| Drug | Countries | |
|---|---|---|
| VIADOX | United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.