VOCINTI Film-coated tablet Ref.[50552] Active ingredients: Vonoprazan
Source: Health Sciences Authority (SG) Revision Year: 2019 Publisher: Manufactured by TAKEDA PHARMACEUTICAL COMPANY LIMITED, Hikari Plant, Yamaguchi, Japan. Repacked by KOKANDO CO. LTD, Toyama, Japan Imported by Takeda (Thailand) Ltd., Bangkok, Thailand
5.1. Pharmacodynamic properties
Mechanism of Action
Vonoprazan is a potassium competitive acid blocker (PCAB) and inhibits H+, K+-ATPase in a reversible and potassium-competitive manner. It does not require activation by acid. Vonoprazan is a strong base with a high affinity for the acid pump of gastric cells inhibiting gastric acid production.
Serum Gastrin and Serum Pepsinogen Effects
Increased serum gastrin and serum pepsinogen concentrations are physiological responses to treatment with acid suppression therapy, including vonoprazan. Increased serum gastrin and serum pepsinogen concentrations were reported with a higher incidence in the vonoprazan treatment groups compared with lansoprazole treatment groups. Serum gastrin and serum pepsinogen concentrations returned to baseline over time upon discontinuation of vonoprazan. The increase in serum gastrin concentration occurred early in treatment with vonoprazan and remained stable for the remainder of treatment.
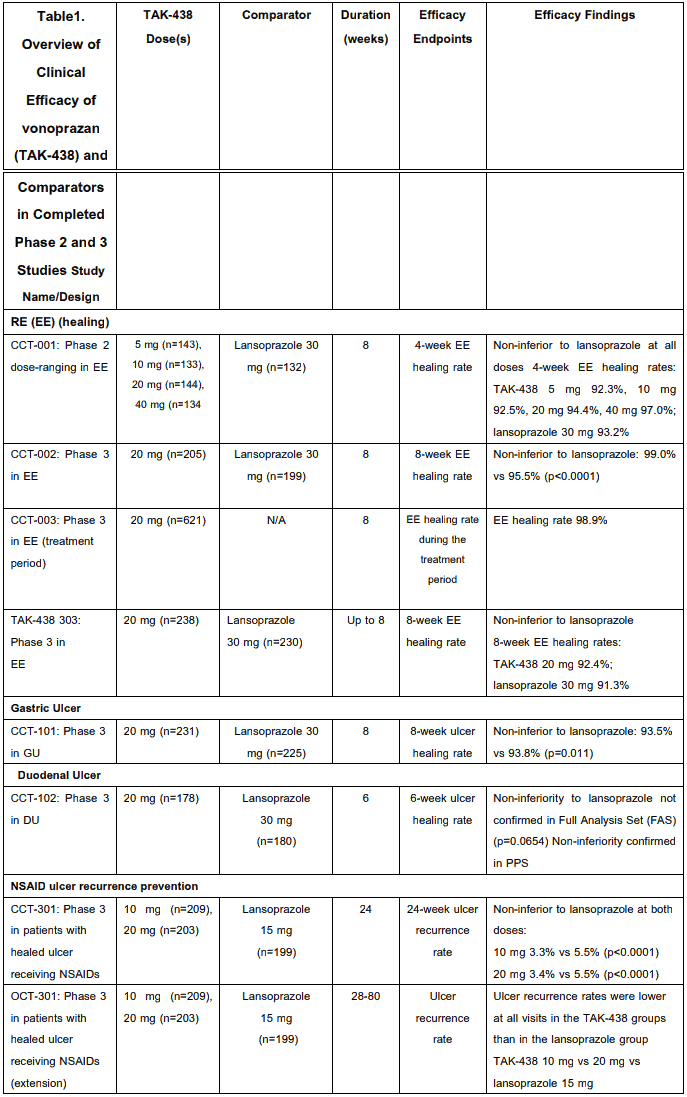
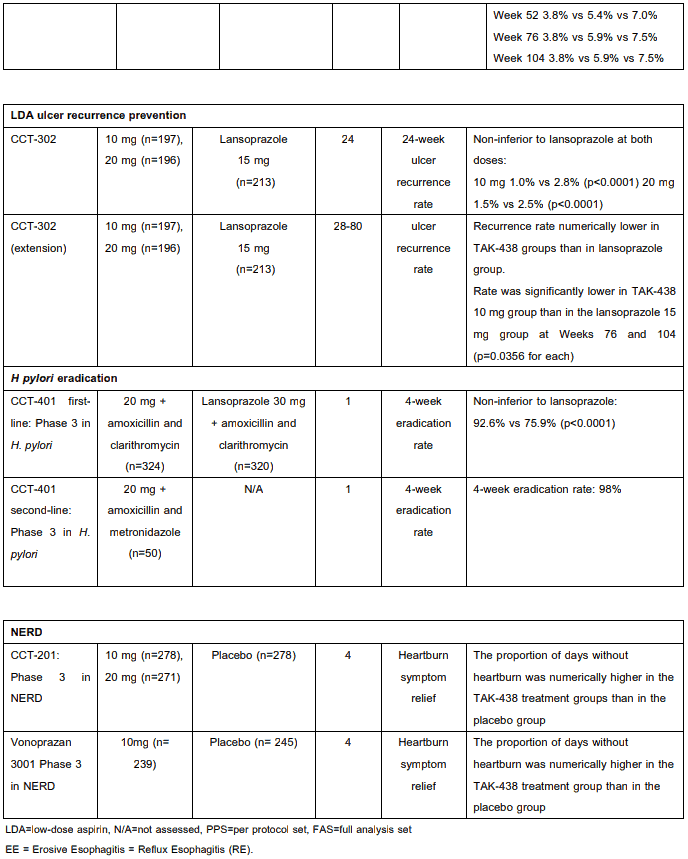
Clinical Studies
The efficacy of vonoprazan has been demonstrated in a number of clinical studies across several indications including GU, DU, RE, prevention of GU/DU during low-dose aspirin or NSAID administration and as an adjunct to H. pylori eradication (see Section 4.1). Clinical efficacy in completed phase 2 and 3 studies is summarized in Table 3. These data are divided into the categories based upon the specific indication, including GU, DU, RE, prevention of recurrence of gastric or duodenal ulcer during low-dose aspirin or NSAID administration, and H. pylori eradication. Following administration of vonoprazan at a dose of 10 mg or 20 mg in healthy adult male subjects for 7 days, pH 4 HTR (pH 4 holding time ratio) (percentage of time pH is maintained at a level ≥4 in 24 hours) was 63±9% and 83±17% respectively.
A phase 1 open-label pharmacodynamics study to investigate the acid-inhibitory effect of vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole sodium 10 mg in healthy adult male Japanese subjects showed that the acid-inhibitory effect of vonoprazan was greater than that of esomeprazole or rabeprazole17). After all treatments, the mean 24-hour pH 4 HTRs increased from Baseline to Day 1 and from Day 1 to Day 7. The mean pH 4 HTRs were higher after administration of vonoprazan on Day 1 than after administration of esomeprazole or rabeprazole on Day 7. The mean 24-hour pH 4 HTRs for vonoprazan and rabeprazole at Baseline were both 8.9%, and on Day 1 and on Day 7 were 84.16% vs 26.29%, and 93.79% vs 65.09%, respectively.
5.2. Pharmacokinetic properties
Following 7 day repeat once daily doses of vonoprazan at doses of 10-40 mg, in healthy adult male subjects, AUCτ,ss and Cmax increase in a slightly greater than dose proportional manner. Steady state has been reached by day 3 of administration, since the trough level of the blood concentration of vonoprazan is constant between day 3 and day 7 of administration.
In addition, vonoprazan does not exhibit time-dependent pharmacokinetics. The following table shows pharmacokinetic parameters of vonoprazan on day 7 of administration.
| Dose | 10 mg | 20 mg |
|---|---|---|
| tmax,ss (h) | 1.5 (0.75, 3.0) | 1.5 (0.75, 3.0) |
| Cmax,ss (ng/mL) | 12.0±1.8 | 23.3±6.6 |
| t1/2Z(h) | 7.0±1.6 | 6.1±1.2 |
| AUCτ,ss (h ng/mL) | 79.5±16.1 | 151.6±40.3 |
Mean ± S.D. of 9 subjects (tmax,ss is expressed by the median (minimum value, maximum value)
Absorption
Absolute bioavailability has not been determined. The pharmacokinetic parameters of vonoprazan following single administration of vonoprazan to healthy adult male subjects at 20 mg under fasting and fed conditions are presented in the table below:
| Dose Condition | Under fasting | After meal |
|---|---|---|
| tmax,ss(h) | 1.5 (1.0, 3.0) | 3.0 (1.0, 4.0) |
| Cmax,ss(ng/mL) | 24.3±6.6 | 26.8±9.6 |
| t1/2Z(h) | 7.7±1.0 | 7.7±1.2 |
| AUC48(h ng/mL) | 222.1±69.7 | 238.3±71.1 |
Mean ± S.D. of 12 subjects (tmax,ss is expressed by the median (minimum value, maximum value))
Distribution
The mean binding rate is 85.2 to 88.0% when [14C] vonoprazan in the range of 0.1 to 10 μg/mL is added to human plasma (in vitro).
Metabolism
Vonoprazan is metabolized mainly by hepatic drug-metabolizing enzyme CYP3A4 and partially by CYP2B6, CYP2C19 and CYP2D6. Vonoprazan is also metabolized by sulfotransferase SULT2A1 (in vitro).
Vonoprazan exhibits time-dependent inhibitory effect on CYP2B6, CYP2C19 and CYP3A4/5 (in vitro). In addition, vonoprazan shows a slight concentration-dependent inductive effect on CYP1A2, but it shows little inductive effect on CYP2B6 and CYP3A4/5 (in vitro).
Excretion and Elimination
When radioactive-labeled drug (15 mg as vonoprazan) is orally administered to healthy adult male subjects, 98.5% of the radioactivity administered is excreted into urine and feces by 168 hours after administration: 67.4% into urine and 31.1% into feces.
Special Populations
Impaired Renal Function
The effect of renal disorders on pharmacokinetics of vonoprazan was evaluated in subjects with normal renal functionand patients with mild, moderate or severe renal disorder and patients with end-stage renal disease (ESRD). When administered a single dose of vonoprazan 20 mg the AUC∞ was higher by 1.3 to 2.4 times and the Cmax higher by 1.2 to 1.8 timesin patients with mild, moderate or severe renal disorder compared to subjects with normal renal function, indicating an increase in vonoprazan exposure with a reduction in renal function.The AUC∞ was higher by 1.3 times and the Cmax higher by 1.2 timesin ESRD patients compared to those in subjects with normal renal function.
Impaired Hepatic Function
The effect of hepatic disorders on pharmacokinetics of vonoprazan was evaluated in subjects with normal hepatic function and patients with mild, moderate or severe hepatic disorder. When administered a single dose of vonoprazan 20 mg, the AUC∞ was higher by 1.2 to 2.6 timesand the Cmax were higher by 1.2 to 1.8 timesin patients with mild, moderate or severe hepatic disorder, compared to subjects with normal hepatic function.
Age, Gender, Race
Vonoprazan has not been studied in patients under 18 years of age.
There are no clinically relevant gender effects of vonoprazan.
No dedicated ethnic comparison studies have been conducted with vonoprazan. The ethnic sensitivity analysis based on the International Conference for Harmonization (ICH) E5 principles was conducted to assess whether the molecular properties of vonoprazan were sensitive to ethnic factor differences, and whether the diagnosis, medical practice, treatment options, and other epidemiological factors for acid-related disorders would vary dramatically in areas other than Japan. It was concluded that vonoprazan is insentitive to ethnic factor differences.
Drug Interactions
Vonoprazan and clarithromycin
Healthy adult male subjects were administered with a single dose of vonoprazan (40 mg), 30 minutes after breakfast on day 1 and day 8, and with repeated dose of clarithromycin 500 mg (potency) 2 times daily 30 minutes before breakfast and dinner on day 3-9. The AUC∞ and Cmax of vonoprazan increased by 1.6 times and 1.4 times, respectively, when concomitantly administered with clarithromycin compared to those of vonoprazan when administered alone.
Vonoprazan, amoxicillin hydrate and clarithromycin
The drug interaction study in healthy adult male subjects administered twice daily with vonoprazan 20 mg, amoxicillin hydrate 750 mg (potency) and clarithromycin 400 mg (potency) concomitantly for 7 days shows no effect on pharmacokinetics of unchanged amoxicillin, however, AUC12 and Cmax of vonoprazan increased by 1.8 times and 1.9 times, respectively, and AUC12 and Cmax of unchanged clarithromycin increased by 1.5 times and 1.6 times, respectively.
Vonoprazan, amoxicillin hydrate and metronidazole
The drug interaction study in healthy adult male subjects administered twice daily with vonoprazan 20 mg, amoxicillin hydrate 750 mg (potency) and metronidazole 250 mg concomitantly for 7 days showed little difference in the pharmacokinetics of vonoprazan, when administered alone or as triple therapy. No difference was observed in the pharmacokinetics of metronidazole or amoxicillin when administered alone or as triple therapy.
Vonoprazan, Bismuth, Clarithromycin and Amoxicillin
The drug interaction study in Helicobacter pylori positive adult subjects administered twice daily vonoprazan 20 mg or lansoprazole 30 mg with tripotassium bismuth dicitrate 600 mg, clarithromycin 500 mg, and amoxicillin 1000 mg concomitantly for 14 days shows the lack of a clinically meaningful effect of vonoprazan on the pharmacokinetics of bismuth compared with lansoprazole.
Vonoprazan and low-dose aspirin or vonoprazan and NSAIDs
The drug interaction study in healthy adult male subjects administered with vonoprazan 40 mg and aspirin 100 mg or NSAID (loxoprofen sodium 60 mg, diclofenac sodium 25 mg or meloxicam 10 mg) concomitantly showed no clear effect of low-dose aspirin or NSAIDs on pharmacokinetics of vonoprazan and of vonoprazan on pharmacokinetics of low-dose aspirin or NSAIDs.
5.3. Preclinical safety data
Carcinogenesis
Vonoprazan was non-carcinogenic in a long term carcinogenicity study in mice when administered the drug daily via oral gavage for up to 2 years at 0.6, 20, 60, and 200 mg/kg/day. Treatment-related tumors, related to exaggerated pharmacology or sepsis-specificity, were noted in the stomach and liver. In the stomach, benign and malignant neuroendocrine cell tumors were observed at ≥20 (males) and ≥60 (females) mg/kg/day and ≥6 (males) and ≥60 (females) mg/kg/day, respectively. In the liver, increased incidences of hepatocellular adenoma and carcinoma were observed at ≥20 (males) and ≥60 (females) mg/kg/day, and at ≥60 (males) and 200 (females) mg/kg/day, respectively. Hyperplasia of the neuroendocrine cells and associated tumors in the stomach may be due to hypergastrinemia as a consequence of inhibiting gastric acid secretion. The hepatocellular tumors are likely rodent-specific findings that are attributed to prolonged induction of hepatic drug-metabolizing enzymes. The NOAEL was <6 mg/kg/day.
Vonoprazan was non-carcinogenic in a long term carcinogenicity study in rats administered the drug via oral gavage at 5, 15, 50, and 150 mg/kg/day. Treatment-related tumors, related to exaggerated pharmacology or species-specificity, were noted in the stomach and liver. In the stomach, benign and malignant neuroendocrine cell tumors were observed at ≥5 mg/kg/day except for malignant neuroendocrine tumor at 50 mg/kg/day (males). In some instances in benign and malignant neuroendocrine cell tumors, tumor cells showed eosinophilic change but these tumors were also judged to be of neuroendocrine cell origin. In the liver, increased incidences of hepatocellular adenoma and carcinoma were observed at ≥50 mg/kg/day except for hepatocellular carcinoma at 50 mg/kg/day (females). Tumor findings in the stomach and liver are believed to be due to hypergastrinemia as a consequence of inhibiting gastric acid secretion and rodent-specific induction of hepatic drugmetabolizing enzymes, respectively. The occurrence of 4 hepatocholangiocellular tumors at ≥50 mg/kg/day (males) were considered to be treatment-related because they were considered to be associated with induction of hepatocellular tumor, but pairwise comparison did not demonstrate a statistically significant effect.
Mutagenicity
Vonoprazan did not exhibit any mutagenic or clastogenic activity in the in vitro Ames assay, in vitro mammalian chromosome aberration assay, and in vivo rat micronucleus assay.
Impairment of Fertility
When administered daily via oral gavage to male and females rats there were no effects on sperm analysis, estrous cycles or number of corpora lutea observed at doses up to 300 mg/kg/dose. Males were administered vonoprazan prior to and during mating and females dosed for 2 weeks pre-mating through Gestation Day (GD) 6. The NOAEL for male and female general toxicity was 30 mg/kg/day and ≥300 mg/kg/day for reproductive function and early embryonic development.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.