VYNDAMAX Capsule Ref.[49665] Active ingredients: Tafamidis
Source: FDA, National Drug Code (US) Revision Year: 2021
Product description
VYNDAQEL (tafamidis meglumine) and VYNDAMAX (tafamidis) contain tafamidis as the active moiety, which is a selective stabilizer of transthyretin.
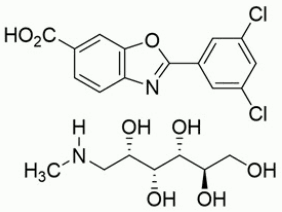
The chemical name of tafamidis meglumine is 2-(3,5-dichlorophenyl)-1,3-benzoxazole-6-carboxylic acid mono (1-deoxy-1-methylamino-D-glucitol). The molecular formula is C14H7Cl2NO3 C7H17NO5, and the molecular weight is 503.33 g/mol. The structural formula is:
Tafamidis meglumine 20-mg soft gelatin capsule for oral use contains a white to pink colored suspension of tafamidis meglumine 20 mg (equivalent to 12.2 mg of tafamidis free acid), and the following inactive ingredients: ammonium hydroxide 28%, brilliant blue FCF, carmine, gelatin, glycerin, iron oxide (yellow), polyethylene glycol 400, polysorbate 80, polyvinyl acetate phthalate, propylene glycol, sorbitan monooleate, sorbitol, and titanium dioxide.
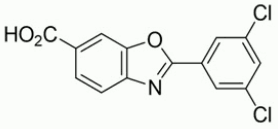
The chemical name of tafamidis is 2-(3,5-dichlorophenyl)-1,3-benzoxazole-6-carboxylic acid. The molecular formula is C14H7Cl2NO3, and the molecular weight is 308.12 g/mol. The structural formula is:
Tafamidis 61-mg soft gelatin capsule for oral use contains a white to pink colored suspension of tafamidis 61 mg and the following inactive ingredients: ammonium hydroxide 28%, butylated hydroxytoluene, gelatin, glycerin, iron oxide (red), polyethylene glycol 400, polysorbate 20, povidone (K-value 90), polyvinyl acetate phthalate, propylene glycol, sorbitol, and titanium dioxide.
| Dosage Forms and Strengths |
|---|
|
VYNDAQEL is available as:
VYNDAMAX is available as:
|
| How Supplied | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
VYNDAQEL 20-mg (tafamidis meglumine) soft gelatin capsules are yellow, opaque, oblong, and printed with "VYN 20" in red and supplied in the following package configurations:
VYNDAMAX 61-mg (tafamidis) soft gelatin capsules are reddish brown, opaque, oblong, and printed with "VYN 61" in white and supplied in the following package configurations:
|
||||||||||||||||||
Drugs
| Drug | Countries | |
|---|---|---|
| VYNDAMAX | Hong Kong, Israel, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.