VYNDAMAX Capsule Ref.[49665] Active ingredients: Tafamidis
Source: FDA, National Drug Code (US) Revision Year: 2021
12.1. Mechanism of Action
Tafamidis is a selective stabilizer of TTR. Tafamidis binds to TTR at the thyroxine binding sites, stabilizing the tetramer and slowing dissociation into monomers, the rate-limiting step in the amyloidogenic process.
12.2. Pharmacodynamics
A proprietary TTR stabilization assay was utilized as a pharmacodynamic marker and assessed the stability of the TTR tetramer ex vivo. The TTR stabilization assay quantifies immunoturbidimetric measurement of the stable TTR tetramer in plasma pre- and post-treatment with 2-day in vitro denaturation with urea. Using this proprietary assay, a dose-dependent trend for greater TTR tetramer stabilization is observed for VYNDAQEL 80-mg compared to VYNDAQEL 20-mg. However, the clinical relevance of a higher TTR tetramer stabilization towards cardiovascular outcomes is not known.
VYNDAQEL stabilized both the wild-type TTR tetramer and the tetramers of 14 TTR variants tested clinically after once-daily dosing. Tafamidis also stabilized the TTR tetramer for 25 variants tested ex vivo.
VYNDAQEL and VYNDAMAX may decrease serum concentrations of total thyroxine, without an accompanying change in thyroid stimulating hormone (TSH). This reduction in total thyroxine values is probably the result of reduced thyroxine binding to or displacement from transthyretin (TTR) due to the high binding affinity of tafamidis to the TTR thyroxine receptor. No corresponding clinical findings consistent with hypothyroidism have been observed.
Biomarkers associated with heart failure (NT-proBNP and Troponin I) favored VYNDAQEL over placebo.
Cardiac Electrophysiology
At approximately 2.2 times the steady state peak plasma concentration (Cmax) at the recommended dose, tafamidis does not prolong the QTc interval to any clinically relevant extent.
12.3. Pharmacokinetics
No clinically significant differences in steady state Cmax and area under the plasma concentration over time curve (AUC) of tafamidis were observed for VYNDAMAX 61-mg capsule compared to VYNDAQEL administered as four 20-mg capsules.
Tafamidis exposure increases proportionally over single (up to 480 mg) or multiple (up to 80 mg) (1 to 6 times the approved recommended dosage) once daily dosing.
The apparent clearance were similar after single and repeated administration of VYNDAQEL 80 mg.
Absorption
Median tafamidis peak concentrations occurred within 4 hours following dosing.
Effect of Food
No clinically significant differences in the pharmacokinetics of tafamidis were observed following administration of a high fat, high calorie meal.
Distribution
The apparent steady state volume of distribution of tafamidis meglumine is 16 liters and 18.5 liters for tafamidis. Plasma protein binding of tafamidis is >99% in vitro. Tafamidis primarily binds to TTR.
Elimination
The mean half-life of tafamidis is approximately 49 hours. The apparent oral clearance of tafamidis meglumine is 0.228 L/h (0.263 L/h for tafamidis). The degree of drug accumulation at steady state after repeated tafamidis daily dosing is approximately 2.5-fold greater than that observed after a single dose.
Metabolism
The metabolism of tafamidis has not been fully characterized. However, glucuronidation has been observed.
Excretion
After a single oral dose of tafamidis meglumine 20 mg, approximately 59% of the dose was recovered in feces (mostly as the unchanged drug) and approximately 22% of the dose was recovered in urine (mostly as the glucuronide metabolite).
Specific Populations
No clinically significant differences in the pharmacokinetics of tafamidis were observed based on age, race/ethnicity (Caucasian and Japanese) or renal impairment.
Patients with Hepatic Impairment
Patients with moderate hepatic impairment (Child-Pugh Score of 7 to 9) had decreased systemic exposure (approximately 40%) and increased clearance (approximately 68%) of tafamidis compared to healthy subjects. As TTR levels are lower in subjects with moderate hepatic impairment than in healthy subjects, the exposure of tafamidis relative to the amount of TTR is sufficient to maintain stabilization of the TTR tetramer in these patients. No clinically significant differences in the pharmacokinetics of tafamidis were observed in patients with mild hepatic impairment (Child Pugh Score of 5 to 6) compared to healthy subjects. The effect of severe hepatic impairment on tafamidis is unknown.
Drug Interaction Studies
Clinical Studies
CYP3A4 substrates: No clinically significant differences in the pharmacokinetics of midazolam (a CYP3A4 substrate) or on the formation of its active metabolite (1-hydroxymidazolam) were observed when a single 7.5-mg dose of midazolam was administered prior to and after a 14-day regimen of VYNDAQEL 20-mg once daily.
BCRP substrates: Tafamidis inhibits breast cancer resistant protein (BCRP). In a clinical study in healthy participants, AUCinf and Cmax of the BCRP substrate rosuvastatin increased by 96.75% and 85.59%, respectively following multiple doses of VYNDAMAX 61 mg daily dosing.
In Vitro Studies
Cytochrome P450 Enzymes: Tafamidis induces CYP2B6 and CYP3A4 and does not induce CYP1A2. Tafamidis does not inhibit CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP3A4/5 or CYP2D6.
UDP glucuronosyltransferase (UGT): Tafamidis inhibits intestinal activities of UGT1A1 but neither induces nor inhibits other UDP glucuronosyltransferase (UGT) systemically.
Transporter Systems: In vitro studies and model predictions show that tafamidis has a low potential to inhibit organic anion transporters OAT1 and OAT3 at clinically relevant concentrations. Tafamidis did not show a potential to inhibit Multi-Drug Resistant Protein (MDR1) (also known as P-glycoprotein; P-gp), organic cation transporter OCT2, multidrug and toxin extrusion transporters MATE1 and MATE2K and, organic anion transporting polypeptide OATP1B1 and OATP1B3.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
There was no evidence of an increased incidence of neoplasia in the transgenic (Tg)-rasH2 mouse following repeated daily administration for 26 weeks at daily doses of 0, 10, 30 or 90 mg/kg. There was no evidence of increased incidence of neoplasia in a 2-year carcinogenicity study in rats at exposures up to 18 times the AUC at the MRHD.
Mutagenesis
There was no evidence of mutagenicity or clastogenicity in vitro, and an in vivo rat micronucleus study was negative.
Impairment of Fertility
There were no effects of tafamidis meglumine on fertility, reproductive performance, or mating behavior in the rat at any dose. Rats were dosed daily (0, 5, 15, and 30 mg/kg/day) prior to cohabitation (for at least 15 days for females and 28 days for males), throughout the cohabitation period to the day prior to termination of males and through to implantation of females (Gestation Day 7). No adverse effects were noted on male and female rats in toxicity, fertility, and mating behavior at any dose. The paternal and maternal no observed adverse effect level for reproductive toxicity of tafamidis meglumine is 30 mg/kg/day, approximately 4 times the MRHD on a mg/m2 basis.
14. Clinical Studies
Efficacy was demonstrated in a multicenter, international, randomized, double-blind, placebo-controlled study in 441 patients with wild-type or hereditary ATTR-CM (NCT01994889).
Patients were randomized in a 1:2:2 ratio to receive VYNDAQEL 20 mg (n=88), VYNDAQEL 80 mg (administered as four 20-mg VYNDAQEL capsules) (n=176), or matching placebo (n=177) once daily for 30 months, in addition to standard of care (e.g., diuretics). Treatment assignment was stratified by the presence or absence of a variant TTR genotype as well as baseline disease severity (NYHA Class). Transplant patients were excluded from this study. Table 1 describes the patient demographics and baseline characteristics.
Table 1. Patient Demographics and Baseline Characteristics:
| Characteristic | Pooled Tafamidis N=264 | Placebo N=177 |
|---|---|---|
| Age - years | ||
| Mean (standard deviation) | 74.5 (7.2) | 74.1 (6.7) |
| Median (minimum, maximum) | 75 (46, 88) | 74 (51, 89) |
| Sex - number (%) | ||
| Male | 241 (91.3) | 157 (88.7) |
| Female | 23 (8.7) | 20 (11.3) |
| TTR Genotype - number (%) | ||
| ATTRm | 63 (23.9) | 43 (24.3) |
| ATTRwt | 201 (76.1) | 134 (75.7) |
| NYHA Class - number (%) | ||
| NYHA Class I | 24 (9.1) | 13 (7.3) |
| NYHA Class II | 162 (61.4) | 101 (57.1) |
| NYHA Class III | 78 (29.5) | 63 (35.6) |
Abbreviations: ATTRm = variant transthyretin amyloid, ATTRwt = wild-type transthyretin amyloid
The primary analysis used a hierarchical combination applying the method of Finkelstein-Schoenfeld (F-S) to all-cause mortality and frequency of cardiovascular-related hospitalizations, which was defined as the number of times a subject was hospitalized (i.e., admitted to a hospital) for cardiovascular-related morbidity. The method compared each patient to every other patient within each stratum in a pair-wise manner that proceeded in a hierarchical fashion using all-cause mortality followed by frequency of cardiovascular-related hospitalizations when patients could not be differentiated based on mortality.
This analysis demonstrated a significant reduction (p=0.0006) in all-cause mortality and frequency of cardiovascular-related hospitalizations in the pooled VYNDAQEL 20-mg and 80-mg groups versus placebo (Table 2).
Table 2. Primary Analysis Using Finkelstein-Schoenfeld (F-S) Method of All-Cause Mortality and Frequency of Cardiovascular-Related Hospitalizations:
| Primary Analysis | Pooled VYNDAQEL N=264 | Placebo N=177 |
|---|---|---|
| Number (%) of Subjects Alive* at Month 30 | 186 (70.5) | 101 (57.1) |
| Mean Number of Cardiovascular-related Hospitalizations During 30 months (per patient per year) Among Those Alive at Month 30 | 0.297 | 0.455 |
| p-value from F-S Method | 0.0006 | |
* Heart transplantation and cardiac mechanical assist device implantation are considered indicators of approaching end stage. As such, these subjects are treated in the analysis as equivalent to death. Therefore, such subjects are not included in the count of "Number of Subjects Alive at Month 30" even if such subjects are alive based on 30 month vital status follow-up assessment.
Analysis of the individual components of the primary analysis (all-cause mortality and cardiovascular-related hospitalization) also demonstrated significant reductions for VYNDAQEL versus placebo.
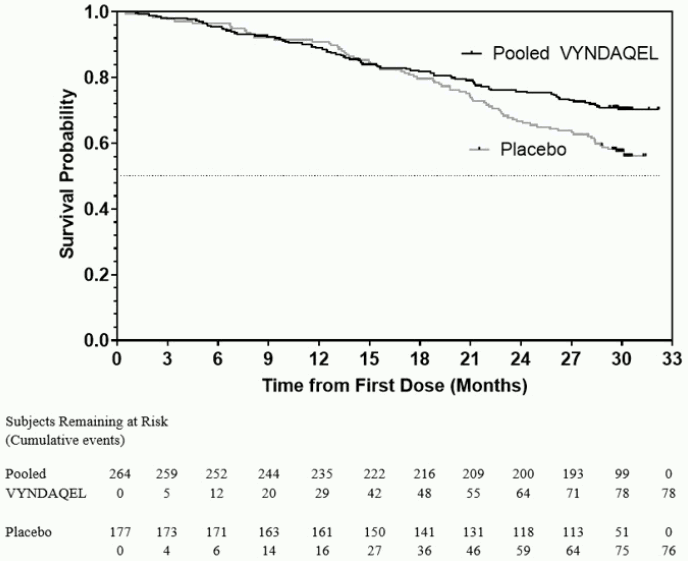
The hazard ratio from the all-cause mortality Cox-proportional hazard model for pooled VYNDAQEL versus placebo was 0.70 (95% confidence interval [CI] 0.51, 0.96), indicating a 30% relative reduction in the risk of death relative to the placebo group (p=0.026). Approximately 80% of total deaths were cardiovascular-related in both treatment groups. A Kaplan-Meier plot of time to event all-cause mortality is presented in Figure 1.
Figure 1. All-Cause Mortality*:
* Heart transplants and cardiac mechanical assist devices treated as death. Hazard ratio from Cox proportional hazards model with treatment, TTR genotype (variant and wild-type), and NYHA baseline classification (NYHA Classes I and II combined and NYHA Class III) as factors.
There were significantly fewer cardiovascular-related hospitalizations with VYNDAQEL compared with placebo with a reduction in risk of 32% corresponding to a Relative Risk Ratio of 0.68 (Table 3).
Table 3. Cardiovascular-Related Hospitalization Frequency:
| Pooled VYNDAQEL N=264 | Placebo N=177 | |
|---|---|---|
| Total (%) Number of Subjects with Cardiovascular-related Hospitalizations | 138 (52.3) | 107 (60.5) |
| Cardiovascular-related Hospitalizations per Year* | 0.48 | 0.70 |
| Pooled VYNDAQEL vs Placebo Treatment Difference (Relative Risk Ratio)* | 0.68 | |
| p-value* | <0.0001 | |
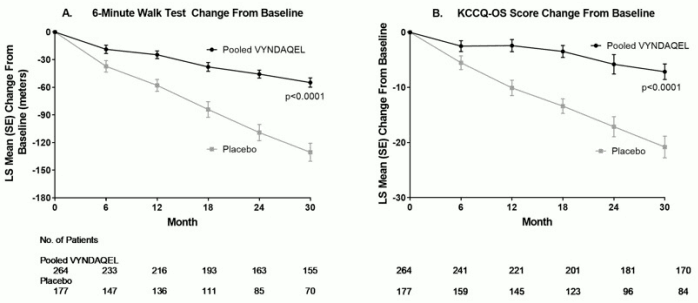
The treatment effects of VYNDAQEL on functional capacity and health status were assessed by the 6-Minute Walk Test (6MWT) and the Kansas City Cardiomyopathy Questionnaire-Overall Summary (KCCQ-OS) score, respectively. A significant treatment effect favoring VYNDAQEL was first observed at Month 6 and remained consistent through Month 30 on both 6MWT distance and KCCQ-OS score (Figure 2 and Table 4).
Figure 2. Change from Baseline to Month 30 in 6MWT Distance and KCCQ-OS Score:
Abbreviations: 6MWT=6-Minute Walk Test, KCCQ-OS=Kansas City Cardiomyopathy Questionnaire-Overall Summary.
Panel A shows change from Baseline to Month 30 in pooled VYNDAQEL patients compared with placebo patients in 6MWT distance.
Panel B shows change from Baseline to Month 30 in pooled VYNDAQEL patients compared with placebo patients in KCCQ-OS score.
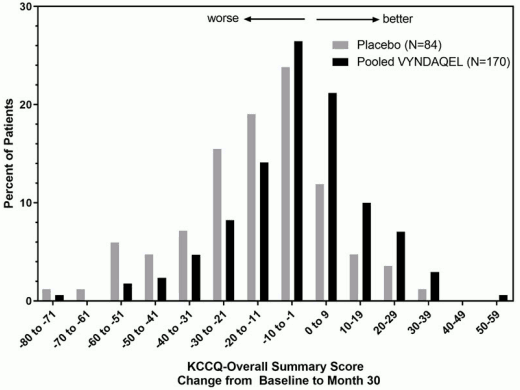
The Kansas City Cardiomyopathy Questionnaire-Overall Summary (KCCQ-OS) score is composed of four domains including Total Symptoms (Symptom Frequency and Symptom Burden), Physical Limitation, Quality of Life, and Social Limitation. The Overall Summary score and domain scores range from 0 to 100, with higher scores representing better health status. All four domains favored pooled VYNDAQEL compared to placebo at Month 30, and demonstrated similar treatment effects to the KCCQ-OS score (Figure 2 and Table 4). The distribution for change from Baseline to Month 30 for KCCQ-OS (Figure 3) shows that the proportion of patients with worse KCCQ-OS scores was lower for the pooled VYNDAQEL-treated group compared to placebo, and the proportion with improved scores was higher (Figure 3).
Figure 3. Histogram of Change from Baseline to Month 30 in KCCQ-Overall Summary Score:
Abbreviation: KCCQ-OS=Kansas City Cardiomyopathy Questionnaire-Overall Summary.
Table 4. 6MWT Distance and KCCQ-OS Scores:
| Endpoints | Baseline Mean (SD) | Change from Baseline to Month 30, LS Mean (SE) | Treatment Difference from Placebo LS Mean (95% CI) | ||
|---|---|---|---|---|---|
| Pooled VYNDAQEL N=264 | Placebo N=177 | Pooled VYNDAQEL | Placebo | ||
| 6MWT (meters) | 351 (121) | 353 (126) | -55 (5) | -131 (10) | 76 (58, 94) |
| KCCQ-OS | 67 (21) | 66 (22) | -7 (1) | -21 (2) | 14 (9, 18) |
Abbreviations: 6MWT = 6-Minute Walk Test; KCCQ-OS = Kansas City Cardiomyopathy Questionnaire-Overall Summary; SD = standard deviation; LS = least squares; SE = standard error; CI = confidence interval
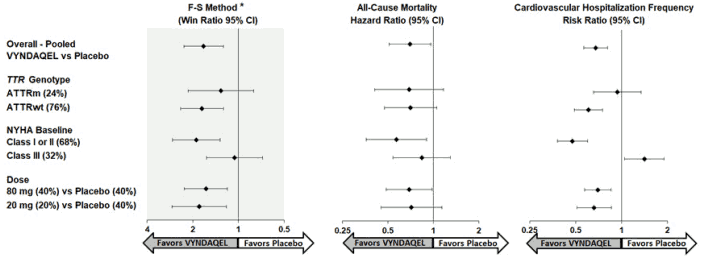
Results from the F-S method represented by win ratio for the combined endpoint and its components (all-cause mortality and frequency of CV-related hospitalization) consistently favored VYNDAQEL versus placebo across all subgroups (wild-type, variant and NYHA Class I & II, and III), except for CV-related hospitalization frequency in NYHA Class III (Figure 4). Win ratio is the number of pairs of VYNDAQEL-treated patient "wins" divided by number of pairs of placebo patient "wins." Analyses of 6MWT and KCCQ-OS also favored VYNDAQEL relative to placebo within each subgroup.
Figure 4. Results by Subgroup, Dose, and Components of Primary Analysis:
Abbreviations: ATTRm = variant transthyretin amyloid, ATTRwt = wild-type transthyretin amyloid, F-S = Finkelstein Schoenfeld, CI = Confidence Interval
*F-S results presented using win ratio (based on all-cause mortality and frequency of cardiovascular hospitalization)
Heart transplants and cardiac mechanical assist devices treated as death.
Results of the primary analysis, 6MWT at Month 30 and KCCQ-OS at Month 30 were statistically significant for both the 80-mg and 20-mg doses of VYNDAQEL vs. placebo, with similar results for both doses.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.