VYVANSE Capsule / Tablet chewable Ref.[11110] Active ingredients: Lisdexamfetamine
Source: FDA, National Drug Code (US) Revision Year: 2019
Product description
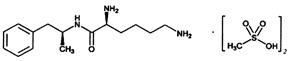
VYVANSE (lisdexamfetamine dimesylate), a CNS stimulant, is for once-a-day oral administration. The chemical designation for lisdexamfetamine dimesylate is (2S)-2,6-diamino-N-[(1S)-1-methyl-2-phenylethyl] hexanamide dimethanesulfonate. The molecular formula is C15H25N3O∙(CH4O3S)2, which corresponds to a molecular weight of 455.60.
The chemical structure is:
Lisdexamfetamine dimesylate is a white to off-white powder that is soluble in water (792 mg/mL).
Information for VYVANSE capsules
VYVANSE capsules contain 10 mg, 20 mg, 30 mg, 40 mg, 50 mg, 60 mg, and 70 mg of lisdexamfetamine dimesylate (equivalent to 5.8 mg, 11.6 mg, 17.3 mg, 23.1 mg, 28.9 mg, 34.7 mg, and 40.5 mg of lisdexamfetamine).
Inactive ingredients: microcrystalline cellulose, croscarmellose sodium, and magnesium stearate. The capsule shells contain gelatin, titanium dioxide, and one or more of the following: FD&C Red #3, FD&C Yellow #6, FD&C Blue #1, Black Iron Oxide, and Yellow Iron Oxide.
Information for VYVANSE chewable tablets
VYVANSE chewable tablets contain 10 mg, 20 mg, 30 mg, 40 mg, 50 mg, and 60 mg of lisdexamfetamine dimesylate (equivalent to 5.8 mg, 11.6 mg, 17.3 mg, 23.1 mg, 28.9 mg, and 34.7 mg of lisdexamfetamine).
Inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, guar gum, magnesium stearate, mannitol, microcrystalline cellulose, sucralose, artificial strawberry flavor.
| Dosage Forms and Strengths |
|---|
|
Information for VYVANSE capsules:
Information for VYVANSE chewable tablets:
|
| How Supplied |
|---|
|
Information for VYVANSE capsules:
Information for VYVANSE chewable tablets:
Manufactured for: Shire US Inc., 300 Shire Way, Lexington, MA 02421 |
Drugs
| Drug | Countries | |
|---|---|---|
| VYVANSE | Australia, Canada, Hong Kong, Israel, Japan, New Zealand, Singapore, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.