WILATE Powder and solvent for solution for injection Ref.[50681] Active ingredients: Coagulation factor VIII Von Willebrand factor
Source: Health Products Regulatory Authority (IE) Revision Year: 2021 Publisher: Octapharma (IP) SPRL, Allée de la Recherche 65, 1070 Anderlecht, Belgium
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Antihaemorrhagics: blood coagulation factors Von Willebrand factor and coagulation factor VIII in combination
ATC Code: B02BD06
Von Willebrand disease (VWD)
The VWF (from the concentrate) is a normal constituent of the human plasma and behaves in the same way as endogenous VWF.
Administration of VWF allows correction of the haemostatic abnormalities exhibited in patients who suffer from VWF deficiency (VWD) at two levels:
- VWF re-establishes platelet adhesion to the vascular sub-endothelium at the site of vascular damage (as it binds both to the vascular sub-endothelium and to the platelet membrane), providing primary haemostasis as shown by the shortening of the bleeding time. This effect occurs immediately and is known to depend to a large extent to the level of polymerisation of the protein;
- VWF produces delayed correction of the associated factor VIII deficiency. Administered intravenously, VWF binds endogenous factor VIII (which is produced normally by the patient), and by stabilising this factor, avoids its rapid degradation. Because of this, administration of pure VWF (VWF product with a low factor VIII level) restores the FVIII:C level to normal as a secondary effect after first infusion. Administration of a factor VIII-containing VWF preparation restores the FVIII:C level to normal immediately after first infusion.
In addition to its role as a factor VIII-protecting protein, VWF mediates platelet adhesion to sites of vascular injury and plays a role in platelet aggregation.
Haemophilia A
The factor VIII/von Willebrand factor complex consists of two molecules (factor VIII and von Willebrand factor) with different physiological functions. When infused into a haemophiliac patient, factor VIII binds to von Willebrand factor in the patient´s circulation. Activated factor VIII acts as a cofactor for activated factor IX, accelerating the conversion of factor X to activated factor X. Activated factor X converts prothrombin into thrombin. Thrombin then converts fibrinogen into fibrin and a clot can be formed.
Haemophilia A is a sex-linked hereditary disorder of blood coagulation due to decreased levels of FVIII:C and results in profuse bleeding into joints, muscles or internal organs, either spontaneously or as results of accidental or surgical trauma. By replacement therapy the plasma levels of factor VIII are increased, thereby enabling a temporary correction of the factor deficiency and correction of the bleeding tendencies.
Of note, annualized bleeding rate (ABR) is not comparable between different factor concentrates and between different clinical studies.
In addition to its role as a factor VIII protecting protein, von Willebrand factor mediates platelet adhesion to sites of vascular injury and plays a role in platelet aggregation.
5.2. Pharmacokinetic properties
Von Willebrand disease (VWD)
VWF (from the concentrate) is a normal constituent of the human plasma and acts like the endogenous VWF.
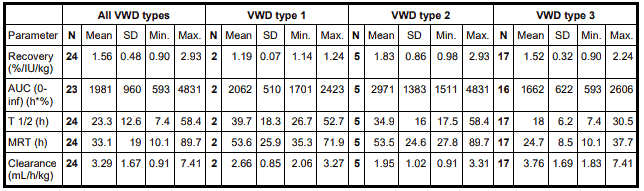
Based on meta-analysis of three pharmacokinetic studies involving 24 evaluable patients with all VWD types, the following results were observed.
Key: AUC = area under the curve; MRT = mean residence time
Haemophilia A
Factor VIII (from the concentrate) is a normal constituent of the human plasma and acts like the endogenous factor VIII. After injection of the product, approximately two thirds to three quarters of the factor VIII remain in the circulation. The level of factor VIII activity reached in the plasma should be between 80-120% of the predicted factor VIII activity.
Plasma factor VIII activity decreases by a two-phase exponential decay. In the initial phase, distribution between the intravascular and other compartments (body fluids) occurs with a half-life of elimination from the plasma of 3 to 6 hours. In the subsequent slower phase, the half-life varies between 8 to 18 hours, with an average of 15 hours. This corresponds to the true biological half-life.
The following results were observed in one clinical study in 12 patients (chromogenic assay, double measurement):
| Parameter | Baseline visit | 6-month visit | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Recovery %/IU/kg | FVIII:C 2.27 | 1.20 | FVIII:C 2.26 | 1.19 |
| AUC~norm~ % * h/IU/kg | FVIII:C 31.3 | 7.31 | FVIII:C 33.8 | 10.9 |
| Half-life (h) | FVIII:C 11.2 | 2.85 | FVIII:C 11.8 | 3.37 |
| MRT (h) | FVIII:C 15.3 | 3.5 | FVIII:C 16.3 | 4.6 |
| Clearance mL/h/kg | FVIII:C 3.37 | 0.86 | FVIII:C 3.24 | 1.04 |
Key: AUC = area under the curve; MRT = mean residence time; SD = standard deviation
5.3. Preclinical safety data
VWF and FVIII in Wilate are normal constituents of the human plasma and act like the endogenous VWF/FVIII.
Conventional safety testing of these compounds in laboratory animals would not add useful information to the existing clinical experience and therefore is not required.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.