XALUPRINE Oral suspension Ref.[8109] Active ingredients: Mercaptopurine
Source: European Medicines Agency (EU) Revision Year: 2019 Publisher: Nova Laboratories Ireland Limited, 3 rd Floor, Ulysses House, Foley Street, Dublin 1, D01 W2T2, Ireland
Therapeutic indications
Xaluprine is indicated for the treatment of acute lymphoblastic leukaemia (ALL) in adults, adolescents and children.
Posology and method of administration
Xaluprine treatment should be supervised by a physician or other healthcare professionals experienced in the management of patients with ALL.
Posology
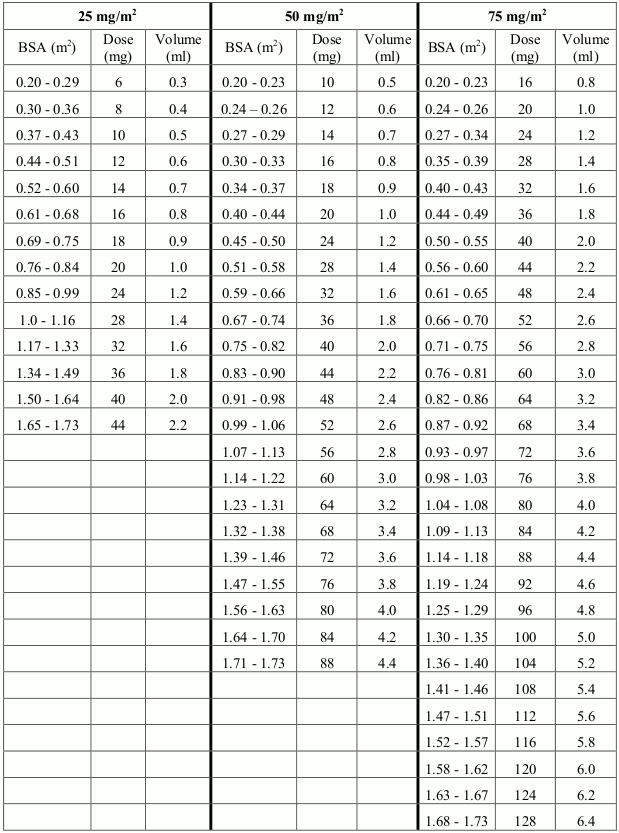
The dose is governed by cautiously monitored haematotoxicity and the dose should be carefully adjusted to suit the individual patient in accordance with the employed treatment protocol. Depending on phase of treatment, starting or target doses generally vary between 25-75 mg/m² body surface area (BSA) per day, but should be lower in patients with reduced or absent Thiopurine Methyl Transferase (TPMT) enzyme activity (see section 4.4).
6-mercaptopurine is metabolised by the polymorphic TPMT enzyme. Patients with little or no inherited TPMT activity are at increased risk for severe toxicity from conventional doses of mercaptopurine and generally require substantial dose reduction. TPMT genotyping or phenotyping can be used to identify patients with absent or reduced TPMT activity. TPMT testing cannot substitute for haematological monitoring in patients receiving Xaluprine. The optimal starting dose for homozygous deficient patients has not been established (see section 4.4).
Special populations
Elderly
No specific studies have been carried out in the elderly. However, it is advisable to monitor renal and hepatic function in these patients, and if there is any impairment, consideration should be given to reducing the Xaluprine dose.
Renal impairment
Since 6-mercaptopurine pharmacokinetics has not been formally studied in renal impairment, no specific dose recommendations can be given. Since impaired renal function may result in slower elimination of mercaptopurine and its metabolites and therefore a greater cumulative effect, consideration should be given to reduced starting doses in patients with impaired renal function. Patients should be closely monitored for dose related adverse reactions.
Hepatic impairment
Since 6-mercaptopurine pharmacokinetics has not been formally studied in hepatic impairment, no specific dose recommendations can be given. Since there is a potential for reduced elimination of mercaptopurine, consideration should be given to reduced starting doses in patients with impaired hepatic function. Patients should be closely monitored for dose related adverse reactions (see section 4.4).
Switching between tablet and oral suspension and vice versa
A tablet form of 6-mercaptopurine is also available. The 6-mecaptopurine oral suspension and tablet are not bioequivalent with respect to peak plasma concentration, and therefore intensified haematological monitoring of the patient is advised on switching formulations (see section 5.2).
Combination with xanthine oxidase inhibitors
Allopurinol and other xanthine oxidase inhibitors decrease the rate of catabolism of 6-mercaptopurine. When allopurinol and 6-mercaptopurine are administered concomitantly it is essential that only a quarter of the usual dose of 6-mercaptopurine is given. Other xanthine oxidase inhibitors should be avoided (see section 4.5).
Patients with NUDT15 variant
Patients with inherited mutated NUDT15 gene are at increased risk for severe 6-mercaptopurine toxicity, (see 4.4). These patients generally require dose reduction; particularly those being NUDT15 variant homozygotes (see 4.4). Genotypic testing of NUDT15 variants may be considered before initiating 6-mercaptopurine therapy. In any case, close monitoring of blood counts is necessary.
Method of administration
Xaluprine is for oral use and requires redispersing (by shaking vigorously at least for 30 seconds) prior to dosing.
Two dosing syringes (a purple syringe graduated to 1 ml and a white syringe graduated to 5 ml) are provided for accurate measurement of the prescribed dose of the oral suspension. It is recommended that the healthcare professional advises the patient or carer which syringe to use to ensure that the correct volume is administered.
Xaluprine may be taken with food or on an empty stomach, but patients should standardise the method of administration. The dose should not be taken with milk or dairy products (see section 4.5). Xaluprine should be taken at least 1 hour before or 2 hours after milk or dairy products.
6-mercaptopurine displays diurnal variation in pharmacokinetics and efficacy. Administration in the evening compared to morning administration may lower the risk of relapse. Therefore the daily dose of Xaluprine should be taken in the evening.
To assist accurate and consistent dose delivery to the stomach water should be taken after each dose of Xaluprine.
Overdose
Symptoms and signs
Gastrointestinal effects, including nausea, vomiting and diarrhoea and anorexia may be early symptoms of overdose having occurred. The principal toxic effect is on the bone marrow, resulting in myelosuppression. Haematological toxicity is likely to be more profound with chronic overdose than with a single ingestion of Xaluprine. Liver dysfunction and gastroenteritis may also occur. The risk of overdose is also increased when xanthine oxidase inhibitors is being given concomitantly with 6-mercaptopurine (see section 4.5).
Management
As there is no known antidote the blood picture should be closely monitored and general supportive measures, together with appropriate blood transfusion, instituted if necessary. Active measures (such as the use of activated charcoal or gastric lavage) may not be effective in the event of 6-mercaptopurine overdose unless the procedure can be undertaken within 60 minutes of ingestion.
Shelf life
15 months.
After first opening: 56 days.
Special precautions for storage
Do not store above 25°C.
Keep the bottle tightly closed (see section 6.6).
Nature and contents of container
Amber type III glass bottle with tamper evident child-resistant closure (HDPE with expanded polyethylene liner) containing 100 ml of oral suspension.
Each pack contains one bottle, an HDPE bottle adaptor and 2 polyethylene dosing syringes (a purple syringe graduated to 1 ml and a white syringe graduated to 5 ml).
Special precautions for disposal and other handling
Safe handling
Anyone handling Xaluprine should wash their hands before and after administering a dose. To decrease the risk of exposure, parents and care givers should wear disposable gloves when handling Xaluprine.
Xaluprine contact with skin or mucous membrane must be avoided. If Xaluprine comes into contact with skin or mucosa, it should be washed immediately and thoroughly with soap and water. Spillages must be wiped immediately.
Women who are pregnant, planning to be or breast-feeding should not handle Xaluprine.
Parents/care givers and patients should be advised to keep Xaluprine out of the reach and sight of children, preferably in a locked cupboard. Accidental ingestion can be lethal for children.
Keep the bottle tightly closed to protect the integrity of the product and minimise the risk of accidental spillage.
The bottle should be shaken vigorously for at least 30 seconds to ensure the oral suspension is well mixed.
Disposal
Xaluprine is cytotoxic. Any unused product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.