XOPENEX Inhalation solution Ref.[50739] Active ingredients: Salbutamol
Source: FDA, National Drug Code (US) Revision Year: 2022
Product description
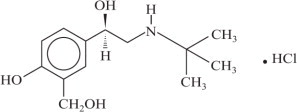
XOPENEX Inhalation Solution is a sterile, clear, colorless, preservative-free solution of the hydrochloride salt of levalbuterol, the (R)-enantiomer of the drug substance racemic albuterol. Levalbuterol HCl is a relatively selective beta2adrenergic receptor agonist [see Clinical Pharmacology (12)]. The chemical name for levalbuterol HCl is (R)α 1-[[(1,1-dimethylethyl) amino]methyl]-4-hydroxy-1,3-benzenedimethanol hydrochloride, and its established chemical structure is as follows:
The molecular weight of levalbuterol HCl is 275.8, and its empirical formula is C13H21NO3•HCl. It is a white to off-white, crystalline solid, with a melting point of approximately 187°C and solubility of approximately 180 mg/mL in water.
Levalbuterol HCl is the USAN modified name for (R)-albuterol HCl in the United States.
XOPENEX Inhalation Solution is supplied in unit-dose vials and requires no dilution before administration by nebulization. Each 3 mL unit-dose vial contains 0.31 mg of levalbuterol (as 0.36 mg of levalbuterol HCl) or 0.63 mg of levalbuterol (as 0.73 mg of levalbuterol HCl) or 1.25 mg of levalbuterol (as 1.44 mg of levalbuterol HCl), sodium chloride to adjust tonicity, and sulfuric acid to adjust the pH to 4.0 (3.3 to 4.5).
| Dosage Forms and Strengths |
|---|
|
Inhalation Solution 3 mL unit-dose, vials in three dosage strengths of levalbuterol; 0.31 mg, 0.63 mg, 1.25 mg. Each strength of XOPENEX Inhalation Solution is available in a shelf carton containing one or more foil pouches, each containing 12 unit-dose vials. |
| How Supplied |
|---|
|
XOPENEX Inhalation Solution is supplied in 3 mL unit-dose, low-density polyethylene (LDPE) vials as a clear, colorless, sterile, preservative-free, aqueous solution, in three different strengths of levalbuterol (0.31 mg, 0.63 mg, 1.25 mg). Each strength of XOPENEX Inhalation Solution is available in a shelf-carton containing one or more foil pouches, each containing 12 unit-dose LDPE vials. XOPENEX (levalbuterol HCl) Inhalation Solution, 0.31 mg (foil pouch label color green) contains 0.31 mg of levalbuterol (as 0.36 mg of levalbuterol HCl) and is available in cartons of 24 unit-dose LDPE vials (NDC 17478-172-24). XOPENEX (levalbuterol HCl) Inhalation Solution, 0.63 mg (foil pouch label color yellow) contains 0.63 mg of levalbuterol (as 0.73 mg of levalbuterol HCl) and is available in cartons of 24 unit-dose LDPE vials (NDC 17478-173-24). XOPENEX (levalbuterol HCl) Inhalation Solution, 1.25 mg (foil pouch label color red) contains 1.25 mg of levalbuterol (as 1.44 mg of levalbuterol HCl) and is available in cartons of 24 unit-dose LDPE vials (NDC 17478-174-24). XOPENEX Inhalation Solution is also available as a concentrate in individually pouched 0.5 mL unit-dose vials containing 1.25 mg of levalbuterol (NDC 17478-171-30). Distributed by: Akorn, Inc., Lake Forest, IL 60045 Manufactured for: Oak Pharmaceuticals, Inc. |
Drugs
| Drug | Countries | |
|---|---|---|
| XOPENEX | United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.