XOPENEX Inhalation solution Ref.[50739] Active ingredients: Salbutamol
Source: FDA, National Drug Code (US) Revision Year: 2022
12.1. Mechanism of Action
Activation of beta2-adrenergic receptors on airway smooth muscle leads to the activation of adenylate cyclase and to an increase in the intracellular concentration of cyclic-3', 5'-adenosine monophosphate (cyclic AMP). The increase in cyclic AMP is associated with the activation of protein kinase A, which in turn inhibits the phosphorylation of myosin and lowers intracellular ionic calcium concentrations, resulting in muscle relaxation. Levalbuterol relaxes the smooth muscles of all airways, from the trachea to the terminal bronchioles. Increased cyclic AMP concentrations are also associated with the inhibition of release of mediators from mast cells in the airway. Levalbuterol acts as a functional antagonist to relax the airway irrespective of the spasmogen involved, thus protecting against all bronchoconstrictor challenges. While it is recognized that beta2-adrenergic receptors are the predominant receptors on bronchial smooth muscle, data indicate that there are beta-receptors in the human heart, 10% to 50% of which are beta2-adrenergic receptors. The precise function of these receptors has not been established [see Warnings and Precautions (5.4)]. However, all beta-adrenergic agonist drugs can produce a significant cardiovascular effect in some patients, as measured by pulse rate, blood pressure, symptoms, and/or electrocardiographic changes.
12.2. Pharmacodynamics
Adults and Adolescents ≥12 Years Old
In a randomized, double-blind, placebo-controlled, cross-over study, 20 adults with mild-to-moderate asthma received single doses of XOPENEX Inhalation Solution (0.31 mg, 0.63 mg, and 1.25 mg) and racemic albuterol sulfate inhalation solution (2.5 mg). All doses of active treatment produced a significantly greater degree of bronchodilation (as measured by percent change from pre-dose mean FEV1) than placebo, and there were no significant differences between any of the active treatment arms. The bronchodilator responses to 1.25 mg of XOPENEX Inhalation Solution and 2.5 mg of racemic albuterol sulfate inhalation solution were clinically comparable over the 6-hour evaluation period, except for a slightly longer duration of action (>15% increase in FEV1 from baseline) after administration of 1.25 mg of XOPENEX Inhalation Solution. Systemic beta-adrenergic adverse effects were observed with all active doses and were generally dose-related for (R)-albuterol. XOPENEX Inhalation Solution at a dose of 1.25 mg produced a slightly higher rate of systemic beta-adrenergic adverse effects than the 2.5 mg dose of racemic albuterol sulfate inhalation solution.
In a randomized, double-blind, placebo-controlled, cross-over study, 12 adults with mild-to-moderate asthma were challenged with inhaled methacholine chloride 20 and 180 minutes following administration of a single dose of 2.5 mg of racemic albuterol sulfate, 1.25 mg of XOPENEX, 1.25 mg of (S)-albuterol, or placebo using a Pari LC Jet nebulizer. Racemic albuterol sulfate, XOPENEX, and (S)-albuterol had a protective effect against methacholine-induced bronchoconstriction 20 minutes after administration, although the effect of (S)-albuterol was minimal. At 180 minutes after administration, the bronchoprotective effect of 1.25 mg of XOPENEX was comparable to that of 2.5 mg of racemic albuterol sulfate. At 180 minutes after administration, 1.25 mg of (S)-albuterol had no bronchoprotective effect.
In a clinical study in adults with mild-to-moderate asthma, comparable efficacy (as measured by change from baseline FEV1) and safety (as measured by heart rate, blood pressure, ECG, serum potassium, and tremor) were demonstrated after a cumulative dose of 5 mg of XOPENEX Inhalation Solution (four consecutive doses of 1.25 mg administered every 30 minutes) and 10 mg of racemic albuterol sulfate inhalation solution (four consecutive doses of 2.5 mg administered every 30 minutes).
12.3. Pharmacokinetics
Adults and Adolescents ≥12 Years Old
The inhalation pharmacokinetics of XOPENEX Inhalation Solution were investigated in a randomized cross-over study in 30 healthy adults following administration of a single dose of 1.25 mg and a cumulative dose of 5 mg of XOPENEX Inhalation Solution and a single dose of 2.5 mg and a cumulative dose of 10 mg of racemic albuterol sulfate inhalation solution by nebulization using a PARI LC Jet nebulizer with a Dura-Neb 2000 compressor.
Following administration of a single 1.25 mg dose of XOPENEX Inhalation Solution, exposure to (R)-albuterol (AUC of 3.3 ng•hr/mL) was approximately 2-fold higher than following administration of a single 2.5 mg dose of racemic albuterol inhalation solution (AUC of 1.7 ng•hr/mL) (see Table 5). Following administration of a cumulative 5 mg dose of XOPENEX Inhalation Solution (1.25 mg given every 30 minutes for a total of four doses) or a cumulative 10 mg dose of racemic albuterol inhalation solution (2.5 mg given every 30 minutes for a total of four doses), Cmax and AUC of (R)-albuterol were comparable (see Table 6).
Table 6. Mean (SD) Values for Pharmacokinetic Parameters in Healthy Adults:
| Single Dose | Cumulative Dose | |||
|---|---|---|---|---|
| XOPENEX 1.25 mg | Racemic albuterol sulfate 2.5 mg | XOPENEX 5 mg | Racemic albuterol sulfate 10 mg | |
| Cmax (ng/mL) (R)-albuterol | 1.1 (0.45) | 0.8 (0.41)** | 4.5 (2.20) | 4.2 (1.51)** |
| Tmax (h)γ (R)-albuterol | 0.2 (0.17, 0.37) | 0.2 (0.17, 1.50) | 0.2 (–0.18*, 1.25) | 0.2 (–0.28*, 1.00) |
| AUC (ng•hr/mL) (R)-albuterol | 3.3 (1.58) | 1.7 (0.99)** | 17.4 (8.56) | 16.0 (7.12)** |
| T½ (h) (R)-albuterol | 3.3 (2.48) | 1.5 (0.61) | 4.0 (1.05) | 4.1 (0.97) |
γ Median (Min, Max) reported for Tmax
* A negative Tmax indicates Cmax occurred between first and last nebulizations.
** Values reflect only (R)-albuterol and do not include (S)-albuterol.
Children 6 to 11 Years Old
The pharmacokinetic parameters of (R)- and (S)-albuterol in children with asthma were obtained using population pharmacokinetic analysis. These data are presented in Table 7. For comparison, adult data obtained by conventional pharmacokinetic analysis from a different study also are presented in Table 7.
In children, AUC and Cmax of (R)-albuterol following administration of 0.63 mg XOPENEX Inhalation Solution were comparable to those following administration of 1.25 mg racemic albuterol sulfate inhalation solution.
When the same dose of 0.63 mg of XOPENEX Inhalation Solution was given to children and adults, the predicted Cmax of (R)-albuterol in children was similar to that in adults (0.52 vs. 0.56 ng/mL), while predicted AUC in children (2.55 ng•hr/mL) was about 1.5-fold higher than that in adults (1.65 ng•hr/mL). These data support lower doses for children 6 to 11 years old compared with the adult doses [see Dosage and Administration (2)].
Table 7. (R)-Albuterol Exposure in Adults and Pediatric Subjects (6 to 11 years):
| Children 6 to 11 years | Adults ≥12 years | |||||
|---|---|---|---|---|---|---|
| Treatment | XOPENEX 0.31 mg | XOPENEX 0.63 mg | Racemic albuterol 1.25 mg | Racemic albuterol 2.5 mg | XOPENEX 0.63 mg | XOPENEX 1.25 mg |
| AUC0-∞ (ng•hr/mL)c | 1.36 | 2.55 | 2.65 | 5.02 | 1.65a | 3.3b |
| Cmax (ng/mL) d | 0.303 | 0.521 | 0.553 | 1.08 | 0.56a | 1.1b |
a The values are predicted by assuming linear pharmacokinetics
b The data obtained from Table 6
c Area under the plasma concentration curve from time 0 to infinity
d Maximum plasma concentration
Metabolism and Elimination
Information available in the published literature suggests that the primary enzyme responsible for the metabolism of albuterol enantiomers in humans is SULT1A3 (sulfotransferase). When racemic albuterol was administered either intravenously or via inhalation after oral charcoal administration, there was a 3- to 4-fold difference in the area under the concentration-time curves between the (R)- and (S)-albuterol enantiomers, with (S)-albuterol concentrations being consistently higher. However, without charcoal pretreatment, after either oral or inhalation administration the differences were 8- to 24-fold, suggesting that (R)-albuterol is preferentially metabolized in the gastrointestinal tract, presumably by SULT1A3.
The primary route of elimination of albuterol enantiomers is through renal excretion (80% to 100%) of either the parent compound or the primary metabolite. Less than 20% of the drug is detected in the feces. Following intravenous administration of racemic albuterol, between 25% and 46% of the (R)-albuterol fraction of the dose was excreted as unchanged (R)-albuterol in the urine.
Special Populations
Hepatic Impairment
The effect of hepatic impairment on the pharmacokinetics of XOPENEX Inhalation Solution has not been evaluated.
Renal Impairment
The effect of renal impairment on the pharmacokinetics of racemic albuterol was evaluated in 5 subjects with creatinine clearance of 7 to 53 mL/min, and the results were compared with those from healthy volunteers. Renal disease had no effect on the half-life, but there was a 67% decline in racemic albuterol clearance. Caution should be used when administering high doses of XOPENEX Inhalation Solution to patients with renal impairment [see Use in Specific Populations (8.6)].
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Although there have been no carcinogenesis studies with levalbuterol HCl, racemic albuterol sulfate has been evaluated for its carcinogenic potential.
In a 2-year study in Sprague-Dawley rats, dietary administration of racemic albuterol sulfate resulted in a significant dose-related increase in the incidence of benign leiomyomas of the mesovarium at doses of 2 mg/kg/day and greater (approximately 4 times the MRHDID of levalbuterol HCl for adults and approximately 5 times the MRHDID of levalbuterol HCl for children on a mg/m² basis). In an 18-month study in CD-1 mice and a 22-month study in the golden hamster, dietary administration of racemic albuterol sulfate showed no evidence of tumorigenicity. Dietary doses in CD-1 mice were up to 500 mg/kg/day (approximately 540 times the MRHDID of levalbuterol HCl for adults and approximately 630 times the MRHDID of levalbuterol HCl for children on a mg/m² basis) and doses in the golden hamster study were up to 50 mg/kg/day (approximately 90 times the MRHDID of levalbuterol HCl for adults on a mg/m² basis and approximately 105 times the MRHDID of levalbuterol HCl for children on a mg/m² basis).
Levalbuterol HCl was not mutagenic in the Ames test or the CHO/HPRT Mammalian Forward Gene Mutation Assay. Levalbuterol HCl was not clastogenic in the in vivo micronucleus test in mouse bone marrow. Racemic albuterol sulfate was not clastogenic in an in vitro chromosomal aberration assay in CHO cell cultures.
No fertility studies have been conducted with levalbuterol hydrochloride. Reproduction studies in rats using racemic albuterol sulfate demonstrated no evidence of impaired fertility at oral doses up to 50 mg/kg/day (approximately 108 times the maximum recommended daily inhalation dose of levalbuterol HCl for adults on a mg/m² basis).
14. Clinical Studies
Adults and Adolescents ≥12 Years Old
The safety and efficacy of XOPENEX Inhalation Solution were evaluated in a 4-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study in 362 adult and adolescent patients 12 years of age and older, with mild-to-moderate asthma (mean baseline FEV1 60% of predicted). Approximately half of the patients were also receiving inhaled corticosteroids. Patients were randomized to receive XOPENEX 0.63 mg, XOPENEX 1.25 mg, racemic albuterol sulfate 1.25 mg, racemic albuterol sulfate 2.5 mg, or placebo three times a day administered via a PARI LC Plus nebulizer and a Dura-Neb portable compressor. Racemic albuterol delivered by a chlorofluorocarbon (CFC) metered-dose inhaler (MDI) was used on an as-needed basis as the rescue medication.
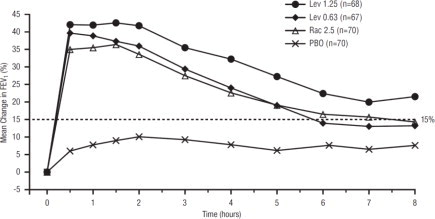
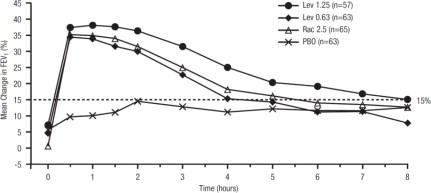
Efficacy, as measured by the mean percent change from baseline FEV1, was demonstrated for all active treatment regimens compared with placebo on day 1 and day 29. On both day 1 (see Figure 1) and day 29 (see Figure 2), 1.25 mg of XOPENEX demonstrated the largest mean percent change from baseline FEV1 compared with the other active treatments. A dose of 0.63 mg of XOPENEX and 2.5 mg of racemic albuterol sulfate produced a clinically comparable mean percent change from baseline FEV1 on both day 1 and day 29.
Figure 1. Mean Percent Change from Baseline FEV1 on Day 1, Adults and Adolescents ≥12 years old:
Figure 2. Mean Percent Change from Baseline FEV1 on Day 29, Adults and Adolescents ≥12 years old:
The mean time to onset of a 15% increase in FEV1 over baseline for levalbuterol at doses of 0.63 mg and 1.25 mg was approximately 17 minutes and 10 minutes, respectively, and the mean time to peak effect for both doses was approximately 1.5 hours after 4 weeks of treatment. The mean duration of effect, as measured by a >15% increase from baseline FEV1, was approximately 5 hours after administration of 0.63 mg of levalbuterol and approximately 6 hours after administration of 1.25 mg of levalbuterol after 4 weeks of treatment. In some patients, the duration of effect was as long as 8 hours.
Children 6 to 11 Years Old
A multicenter, randomized, double-blind, placebo- and active-controlled study was conducted in children with mild-to-moderate asthma (mean baseline FEV1 73% of predicted) (n=316). Following a 1-week placebo run-in, subjects were randomized to XOPENEX (0.31 or 0.63 mg), racemic albuterol (1.25 or 2.5 mg), or placebo, which were delivered three times a day for 3 weeks using a PARI LC Plus nebulizer and a Dura-Neb 3000 compressor.
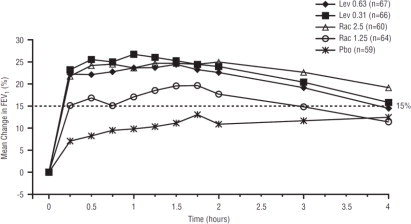
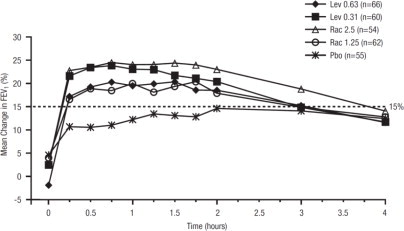
Efficacy, as measured by mean peak percent change from baseline FEV1, was demonstrated for all active treatment regimens compared with placebo on day 1 and day 21. Time profile FEV1 curves for day 1 and day 21 are shown in Figure 3 and Figure 4, respectively. The onset of effect (time to a 15% increase in FEV1 over test-day baseline) and duration of effect (maintenance of a >15% increase in FEV1 over test-day baseline) of levalbuterol were clinically comparable to those of racemic albuterol.
Figure 3. Mean Percent Change from Baseline FEV1 on Day 1, Children 6 to 11 Years of Age:
Figure 4. Mean Percent Change from Baseline FEV1 on Day 21, Children 6 to 11 Years of Age:
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.