XOSPATA Tablet Ref.[10305] Active ingredients: Gilteritinib
Source: FDA, National Drug Code (US) Revision Year: 2019
Product description
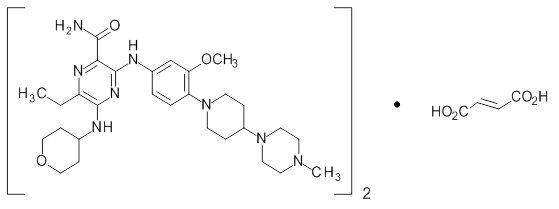
Gilteritinib is a tyrosine kinase inhibitor. The chemical name is 2-Pyrazinecarboxamide, 6-ethyl-3-[[3-methoxy-4-[4-(4-methyl-1-piperazinyl)-1-piperidinyl] phenyl] amino]-5-[(tetrahydro-2Hpyran-4-yl) amino], (2E)-2-butenedioate (2:1). The molecular weight is 1221.50 and the molecular formula is (C29H44N8O3)2·C4H4O4. The structural formula is:
Gilteritinib fumarate is a light yellow to yellow powder or crystals that is sparingly soluble in water and very slightly soluble in anhydrous ethanol.
XOSPATA (gilteritinib) is provided as a tablet for oral administration. Each tablet contains 40 mg of gilteritinib active ingredient as free base (corresponding to 44.2 mg gilteritinib fumarate). The inactive ingredients are ferric oxide, hydroxypropyl cellulose, hypromellose, low-substituted hydroxypropyl cellulose, mannitol, magnesium stearate, talc, polyethylene glycol and titanium dioxide.
| Dosage Forms and Strengths |
|---|
|
Tablets: 40 mg as light yellow, round-shaped, film-coated tablets debossed with the Astellas logo and '235' on the same side. |
| How Supplied |
|---|
|
XOSPATA (gilteritinib) 40 mg tablets are supplied as light yellow, round-shaped, film-coated tablets debossed with the Astellas logo and '235' on the same side. XOSPATA tablets are available in the following package size:
|
Drugs
| Drug | Countries | |
|---|---|---|
| XOSPATA | Austria, Brazil, Canada, Estonia, Finland, France, Croatia, Ireland, Israel, Italy, Japan, Lithuania, Poland, Romania, Turkey, United Kingdom, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.