XULANE Patch Ref.[10487] Active ingredients: 17 alpha-Ethinylestradiol Norelgestromin
Source: FDA, National Drug Code (US) Revision Year: 2020
12.1. Mechanism of Action
NGMN is the active progestin largely responsible for the progestational activity that occurs in women following application of norelgestromin and ethinyl estradiol transdermal system. NGMN is also the primary active metabolite produced following oral administration of NGM, the progestin component of some oral contraceptive products.
Combination hormonal contraceptives act by suppression of gonadotropins. Although the primary mechanism of this action is inhibition of ovulation, other alterations include changes in the cervical mucus (which increase the difficulty of sperm entry into the uterus) and the endometrium (which reduce the likelihood of implantation).
12.2. Pharmacodynamics
One clinical trial assessed the return of hypothalamic-pituitary-ovarian axis function post-therapy and found that follicle-stimulating hormone (FSH), luteinizing hormone (LH), and estradiol mean values, though suppressed during therapy, returned to near baseline values during the 6 weeks post-therapy.
12.3. Pharmacokinetics
Absorption
The systemic delivery rate of NGMN and EE from norelgestromin and ethinyl estradiol transdermal system is approximately 150 mcg of NGMN and 35 mcg of EE per day based on a comparative analysis with intravenous (IV) data. Following a single application of norelgestromin and ethinyl estradiol transdermal system, both NGMN and EE reach a plateau by approximately 48 hours. Pooled data from the 3 clinical studies have demonstrated that steady state is reached within 2 weeks of application. In one of the clinical studies, Css concentrations across all subjects ranged from 0.305 to 1.53 ng/mL for NGMN and from 23 to 137 pg/mL for EE.
Absorption of NGMN and EE following application of norelgestromin and ethinyl estradiol transdermal system to the buttock, upper outer arm, abdomen and upper torso (excluding breast) was examined. While absorption from the abdomen was slightly lower than from other sites, absorption from these anatomic sites was considered to be therapeutically equivalent.
The mean (%CV) PK parameters Css and AUC0-168 for NGMN and EE following a single buttock application of norelgestromin and ethinyl estradiol transdermal system are summarized in Table 4.
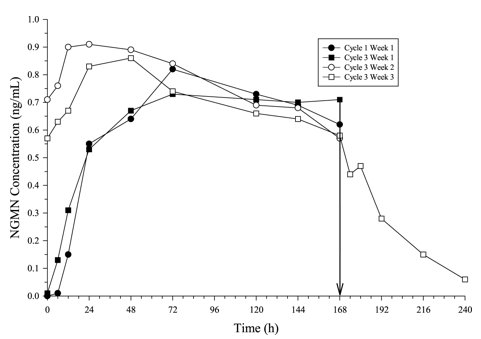
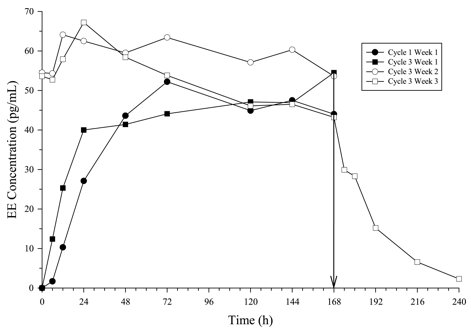
In multiple dose studies, AUC0-168 for NGMN and EE was found to increase over time (Table 4). In a three-cycle study, these PK parameters reached steady state conditions during Cycle 3 (Figures 2 and 3). Upon removal of the patch, serum levels of EE and NGMN reach very low or non-measurable levels within 3 days.
Table 4. Mean (%CV*) PK Parameters of NGMN and EE Following Three Consecutive Cycles of Norelgestromin and Ethinyl Estradiol Transdermal System Wear on the Buttock:
| Analyte | Parameter | Cycle 1 Week 1 | Cycle 3 Week 1 | Cycle 3 Week 2 | Cycle 3 Week 2 |
|---|---|---|---|---|---|
| NGMN | Css (ng/mL) | 0.70 (39.4) | 0.70 (41.8) | 0.80 (28.7) | 0.70 (45.3) |
| AUC0-168 (ng·h/mL) | 107 (44.2) | 105 (43.2) | 132 (43.4) | 120 (43.9) | |
| t1/2(h) | nc | nc | nc | 32.1 (40.3) | |
| EE | Css (pg/mL) | 46.4 (38.5) | 47.6 (36.4) | 59.0 (42.5) | 49.6 (54.4) |
| AUC0-168 (pg·h/mL) | 6796 (39.3) | 7160 (40.4) | 10054 (41.8) | 8840 (58.6) | |
| t1/2 (h) | nc | nc | nc | 21.0 (43.2) |
nc = not calculated
* %CV is % of Coefficient of variation = 100 (standard deviation/mean)
Figure 2. Mean Serum NGMN Concentrations (ng /mL) in Healthy Female Volunteers Following Application of Norelg estromin and Ethinyl Estradiol Transdermal System on the Buttock for Three Cons ecutive Cycles (Vertical arrow indicates time of patch removal):
Figure 3. Mean Serum EE Concentrations (pg /mL) in Healthy Female Volunteers Following Application of Norelg estromin and Ethinyl Estradiol Transdermal System on the Buttock for Three Cons ecutive Cycles (Vertical arrow indicates time of patch removal):
The absorption of NGMN and EE following application of norelgestromin and ethinyl estradiol transdermal system was studied under conditions encountered in a health club (sauna, whirlpool and treadmill) and in a cold water bath. The results indicated that for NGMN, there were no significant treatment effects on Css or AUC when compared to normal wear. For EE, increased exposures were observed due to sauna, whirlpool and treadmill. There was no significant effect of cold water on these parameters.
Results from a study of consecutive norelgestromin and ethinyl estradiol transdermal system wear for 7 days and 10 days indicated that serum concentrations of NGMN and EE dropped slightly during the first 6 hours after the patch replacement, and recovered within 12 hours. By Day 10 of patch administration, both NGMN and EE concentrations had decreased by approximately 25% when compared to Day 7 concentrations.
Metabolism
Since NGMN and EE are delivered transdermally, first-pass metabolism (via the gastrointestinal tract and/or liver) of NGMN and EE that would be expected with oral administration does not occur. Hepatic metabolism of NGMN occurs and metabolites include norgestrel, which is highly bound to SHBG, and various hydroxylated and conjugated metabolites. EE is also metabolized to various hydroxylated products and their glucuronide and sulfate conjugates.
Distribution
NGMN and norgestrel (a serum metabolite of NGMN) are highly bound (>97%) to serum proteins. NGMN is bound to albumin and not to SHBG, while norgestrel is bound primarily to SHBG, which limits its biological activity. EE is extensively bound to serum albumin and induces an increase in the serum concentrations of SHBG (see Table 4).
Elimination
Following removal of patches, the elimination kinetics of NGMN and EE were consistent for all studies with half-life values of approximately 28 hours and 17 hours, respectively. The metabolites of NGMN and EE are eliminated by renal and fecal pathways.
Transdermal versus Oral Contraceptives
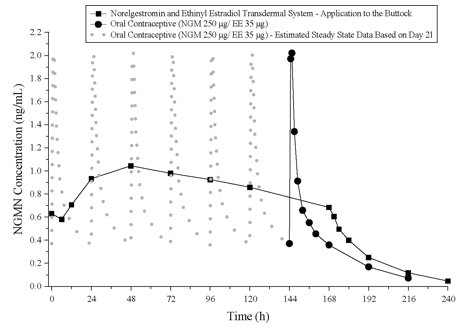
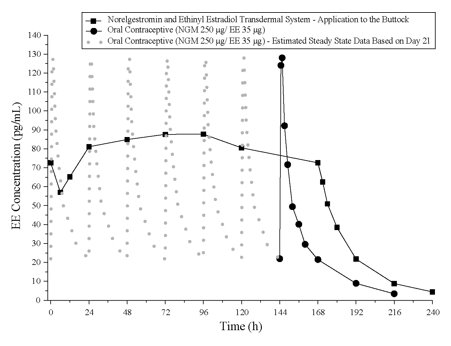
The norelgestromin and ethinyl estradiol transdermal system delivers EE and NGMN over a 7-day period while oral contraceptives (containing NGM 250 mcg / EE 35 mcg) are administered on a daily basis. Figures 4 and 5 present mean PK profiles for EE and NGMN following administration of an oral contraceptive (containing NGM 250 mcg / EE 35 mcg) compared to the 7-day norelgestromin and ethinyl estradiol transdermal system (containing NGMN 4.86 mg / EE 0.53 mg) during Cycle 2 in 32 healthy female volunteers.
Figure 4. Mean Serum Concentration-Time Profiles of NGMN Following Once-Daily Administration of an Oral Contraceptive for Two Cycles or Application of Norelg estromin and Ethinyl Estradiol Transdermal System for Two Cycles to the Buttock in Healthy Female Volunteers. [Oral contraceptive: Cycle 2, Days 15 to 21, Norelg estromin and Ethinyl Estradiol Transdermal System: Cycle 2, Week 3]:
Figure 5. Mean Serum Concentration-Time Profiles of EE Following Once-Daily Administration of an Oral Contraceptive for Two Cycles or Application of Norelg estromin and Ethinyl Estradiol Transdermal System for Two Cycles to the Buttock in Healthy Female Volunteers. [Oral contraceptive: Cycle 2, Days 15 to 21, Norelg estromin and Ethinyl Estradiol Transdermal System: Cycle 2, Week 3]:
Table 5 provides the mean (%CV) for NGMN and EE pharmacokinetic (PK) parameters.
Table 5. Mean (%CV) NGMN and EE Steady State Pharmacokinetic Parameters Following Application of Norelgestromin and Ethinyl Estradiol Transdermal System and Once-Daily Administration of an Oral Contraceptive (containing NGM 250 mcg / EE 35 mcg) in Healthy Female Volunteers:
| Parameter | Norelgestromin and Ethinyl Estradiol Transdermal System* | ORAL CONTRACEPTIVE† |
|---|---|---|
| NGMN‡ | ||
| Cmax (ng/mL) | 1.12 (33.6) | 2.16 (25.2) |
| AUC0-168 (ng·h/mL) | 145 (36.8) | 123 (30.2)§ |
| Css (ng/mL) | 0.888 (36.6) | 0.732 (30.2)¶ |
| EE | ||
| Cmax (pg/mL) | 97.4 (31.6) | 133 (27.7) |
| AUC0-168 (pg·h/mL) | 12,971 (33.1) | 8,281(26.9)§ |
| Css (pg/mL) | 80.0 (33.5) | 49.3 (26.9)¶ |
* Cycle 2, Week 3
† Cycle 2, Day 21
‡ NGM is rapidly metabolized to NGMN following oral administration
§ Average weekly exposure, calculated as AUC24 x 7
¶ Cavg
In general, overall exposure for NGMN and EE (AUC and Css) was higher in subjects treated with norelgestromin and ethinyl estradiol transdermal system for both Cycle 1 and Cycle 2, compared to that for the oral contraceptive, while Cmax values were higher in subjects administered the oral contraceptive. Under steady state conditions, AUC0-168 and Css for EE were approximately 55% and 60% higher, respectively, for the transdermal patch, and the Cmax was about 35% higher for the oral contraceptive, respectively. Inter-subject variability (%CV) for the PK parameters following delivery from norelgestromin and ethinyl estradiol transdermal system was higher relative to the variability determined from the oral contraceptive. The mean PK profiles are different between the two products and caution should be exercised when making a direct comparison of these PK parameters.
In Table 6, percent change in concentrations (%CV) of markers of systemic estrogenic activity (Sex Hormone Binding Globulin [SHBG] and Corticosteroid Binding Globulin [CBG]) from Cycle 1 Day 1 to Cycle 1 Day 22 is presented. Percent change in SHBG concentrations was higher for norelgestromin and ethinyl estradiol transdermal system users compared to women taking the oral contraceptive; percent change in CBG concentrations was similar for norelgestromin and ethinyl estradiol transdermal system and oral contraceptive users. Within each group, the absolute values for SHBG were similar for Cycle 1, Day 22 and Cycle 2, Day 22.
Table 6. Mean Percent Change (%CV) in SHBG and CBG Concentrations Following Once-Daily Administration of an Oral Contraceptive (containing NGM 250 mcg / EE 35 mcg) for One Cycle and Application of Norelgestromin and Ethinyl Estradiol Transdermal System for One Cycle in Healthy Female Volunteers:
| Parameter | Norelgestromin and Ethinyl Estradiol Transdermal System | ORAL CONTRACEPTIVE |
|---|---|---|
| (% change fromDay 1 to Day 22) | (% change fromDay 1 to Day 22) | |
| SHBG | 334 (39.3) | 200 (43.2) |
| CBG | 153 (40.2) | 157 (33.4) |
Drug Interactions
In a PK drug interaction study, oral administration of tetracycline HCl, 500 mg four times daily for 3 days prior to and 7 days during wear of norelgestromin and ethinyl estradiol transdermal system did not significantly affect the PK of NGMN or EE.
Use in Specific Populations
Effects of Age, Body Weight, Body Surface Area and Race
The effects of age, body weight, body surface area and race on the PK of NGMN and EE were evaluated in 230 healthy women from nine pharmacokinetic studies of single 7-day applications of norelgestromin and ethinyl estradiol transdermal system. For both NGMN and EE, increasing age, body weight and body surface area each were associated with slight decreases in Css and AUC values. However, only a small fraction (10% to 25%) of the overall variability in the PK of NGMN and EE following application of norelgestromin and ethinyl estradiol transdermal system may be associated with any or all of the above demographic parameters. There was no significant effect of race with respect to Caucasians, Hispanics and Blacks.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
See Warnings and Precautions (5.3, 5.12) and Use in Specific Populations (8.1).
Norelgestromin was tested in in vitro mutagenicity assays (bacterial plate incorporation mutation assay, CHO/HGPRT mutation assay, chromosomal aberration assay using cultured human peripheral lymphocytes) and in one in vivo test (rat micronucleus assay) and found to have no genotoxic potential.
14. Clinical Studies
In 3 large clinical trials lasting 12 months, in North America, Europe and South Africa, 3,330 women (ages 18 to 45) completed 22,155 cycles of norelgestromin and ethinyl estradiol transdermal system use, the pregnancy rate in women aged 18 to 35 years was 1.07 (95% confidence interval 0.60, 1.76) per 100 woman-years of norelgestromin and ethinyl estradiol transdermal system use. The racial distribution was 91% Caucasian, 4.9% Black, 1.6% Asian, and 2.4% Other.
With respect to weight, 5 of the 15 pregnancies reported with norelgestromin and ethinyl estradiol transdermal system use were among women with a baseline body weight ≥198 lbs., which constituted < 3% of the study population. The greater proportion of pregnancies among women at or above 198 lbs. was statistically significant and suggests that norelgestromin and ethinyl estradiol transdermal system may be less effective in these women.
Patch Adhesion
In the clinical trials with norelgestromin and ethinyl estradiol transdermal system, approximately 2% of the cumulative number of patches completely detached and 3% partially detached. The proportion of subjects with at least one patch that completely detached ranged from 2% to 6%, with a reduction from Cycle 1 (6%) to Cycle 13 (2%). For instructions on how to manage detachment of patches, refer to Dosage and Administration (2).
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.