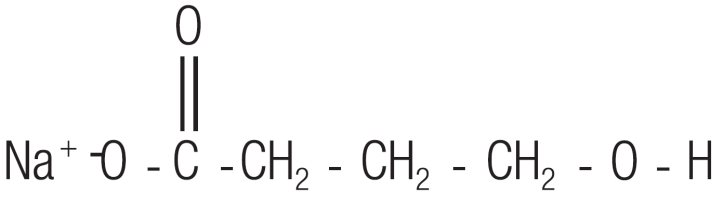XYREM Oral solution Ref.[11068] Active ingredients: Sodium oxybate
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
Sodium oxybate, a CNS depressant, is the active ingredient in Xyrem. The chemical name for sodium oxybate is sodium 4-hydroxybutyrate. The molecular formula is C4H7NaO3, and the molecular weight is 126.09 g/mole.
The chemical structure is:
Sodium oxybate is a white to off-white, crystalline powder that is very soluble in aqueous solutions. Each mL of Xyrem contains 0.5 g of sodium oxybate (equivalent to 0.413 g/mL of oxybate) in USP Purified Water, neutralized to pH 7.5 with malic acid.
| Dosage Forms and Strengths |
|---|
|
Xyrem is a clear to slightly opalescent oral solution, in a concentration of 0.5 g per mL (0.5 g/mL of sodium oxybate equivalent to 0.413 g/mL of oxybate). |
| How Supplied |
|---|
|
Xyrem is a clear to slightly opalescent oral solution. Each prescription includes one bottle of Xyrem with attached press in bottle adaptor, an oral measuring device (plastic syringe), and a Medication Guide. The pharmacy provides two empty containers with child-resistant caps with each Xyrem shipment. Each amber bottle contains Xyrem oral solution at a concentration of 0.5 g per mL (0.5 g/mL of sodium oxybate equivalent to 0.413 g/mL of oxybate) and has a child-resistant cap. One 180 mL bottle NDC 68727-100-01 Distributed By: Jazz Pharmaceuticals, Inc., Palo Alto, CA 94304 |
Drugs
| Drug | Countries | |
|---|---|---|
| XYREM | Austria, Canada, Cyprus, Estonia, Spain, Finland, France, Croatia, Ireland, Italy, Lithuania, Netherlands, Poland, Turkey, United Kingdom, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.