ZULRESSO Solution for injection Ref.[9971] Active ingredients:
Source: FDA, National Drug Code (US) Revision Year: 2019
Product description
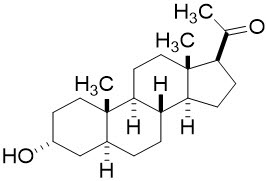
ZULRESSO contains brexanolone, a neuroactive steroid gamma-aminobutyric acid (GABA) A receptor positive modulator, that is chemically identical to endogenous allopregnanolone.
The molecular formula of brexanolone is C21H34O2. The relative molecular mass is 318.5 Da. The chemical structure is:
ZULRESSO (brexanolone) injection is a sterile, clear, colorless, and preservative-free solution. ZULRESSO 5 mg/mL is hypertonic and must be diluted prior to administration as an intravenous infusion [see Dosage and Administration (2.3)]. Each mL of solution contains 5 mg of brexanolone, 250 mg of betadex sulfobutyl ether sodium, 0.265 mg of citric acid monohydrate, 2.57 mg of sodium citrate dihydrate, and water for injection. Hydrochloric acid or sodium hydroxide may be used during manufacturing to adjust pH.
| Dosage Forms and Strengths |
|---|
|
Injection: 100 mg/20 mL (5 mg/mL) clear, colorless solution in a single-dose vial. |
| How Supplied |
|---|
|
ZULRESSO injection is supplied as 100 mg brexanolone in 20 mL (5 mg/mL) single-dose vials containing a sterile, preservative-free, clear, colorless solution. NDC 72152-547-20 |
Drugs
| Drug | Countries | |
|---|---|---|
| ZULRESSO | United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.