ZULRESSO Solution for injection Ref.[9971] Active ingredients:
Source: FDA, National Drug Code (US) Revision Year: 2019
12. Clinical Pharmacology
12.6 Betadex Sulfobutyl Ether Sodium Pharmacokinetics
Betadex sulfobutyl ether sodium is a solubilizing agent in ZULRESSO. In patients with severe renal impairment (eGFR 15-29 mL/minute/1.73 m²), betadex sulfobutyl ether sodium AUCinf increased 5.5-fold and Cmax increased 1.7-fold. Avoid use of ZULRESSO in patients with ESRD [see Use in Specific Populations (8.7)].
12.1. Mechanism of Action
The mechanism of action of brexanolone in the treatment of PPD in adults is not fully understood, but is thought to be related to its positive allosteric modulation of GABAA receptors.
12.2. Pharmacodynamics
Brexanolone potentiated GABA-mediated currents from recombinant human GABAA receptors in mammalian cells expressing α1β2γ2 receptor subunits, α4β3δ receptor subunits, and α6β3δ receptor subunits.
Brexanolone exposure-response relationships and the time course of pharmacodynamics response are unknown.
Cardiac Electrophysiology
The effect of brexanolone on the QT interval was evaluated in a Phase 1 randomized, placebo and positive controlled, double-blind, three-period crossover thorough QT study in 30 healthy adult subjects. At 1.9-times the exposure occurring at the highest recommended infusion rate (90 mcg/kg/hour), brexanolone did not prolong the QT interval to a clinically relevant extent.
12.3. Pharmacokinetics
Brexanolone exhibited dose proportional pharmacokinetics over a dosage range of 30 mcg/kg/hour to 270 mcg/kg/hour (three times the maximum recommended dosage). Mean steady state exposure at 60 mcg/kg/hour and 90 mcg/kg/hour was around 52 ng/mL and 79 ng/mL, respectively.
Distribution
The volume of distribution of brexanolone was approximately 3 L/kg, suggesting extensive distribution into tissues. Plasma protein binding was greater than 99% and is independent of plasma concentrations.
Elimination
The terminal half-life of brexanolone is approximately 9 hours. The total plasma clearance of brexanolone is approximately 1 L/h/kg.
Metabolism
Brexanolone is extensively metabolized by non-CYP based pathways via three main routes - keto-reduction (AKRs), glucuronidation (UGTs), and sulfation (SULTs). There are three major circulating metabolites that are pharmacologically inactive and do not contribute to the overall efficacy of ZULRESSO.
Excretion
Following administration of radiolabeled brexanolone, 47% was recovered in feces (primarily as metabolites) and 42% in urine (with less than 1% as unchanged brexanolone).
Specific Populations
No clinically significant differences in the pharmacokinetics of brexanolone were observed based on renal impairment (severe) study or hepatic impairment (mild, moderate, severe) study. The effect of end stage renal disease (ESRD, eGFR <15 mL/minute/1.73 m²) on brexanolone pharmacokinetics is unknown. However, avoid use of ZULRESSO in patients with ESRD [see Use in Specific Populations (8.7)].
Drug Interaction Studies
No studies were conducted to evaluate the effects of other drugs on ZULRESSO.
No clinically significant differences in the pharmacokinetics of phenytoin (CYP2C9 substrate) were observed when it was used concomitantly with brexanolone.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Carcinogenicity studies of brexanolone have not been performed.
Mutagenesis
Brexanolone was not genotoxic when tested in an in vitro microbial mutagenicity (Ames) assay, an in vitro micronucleus assay in human peripheral blood lymphocytes, and an in vivo rat bone marrow micronucleus assay.
Impairment of Fertility
Treatment of female and male rats with brexanolone at doses equal to and greater than 30 mg/kg/day, which is associated with 2 times the plasma levels at the maximum recommended human dose (MRHD) of 90 mcg/kg/hour, caused impairment of female and male fertility and reproduction. In female rats, brexanolone was associated with decreased mating and fertility indices, an increase in number of days to mating, prolonged/irregular estrous cycles, an increase in the number of early resorptions, and post implantation loss. Reversal of effects in females was observed following a 28-day recovery period. In male rats, brexanolone was associated with decreased mating and fertility indices, decreased conception rate, lower prostate, seminal vesicle, and epididymis weight, as well as decreased sperm numbers. Impaired female and male fertility and reproduction were not observed at 0.8 times the MRHD.
14. Clinical Studies
The efficacy of ZULRESSO in the treatment of postpartum depression (PPD) was demonstrated in two multicenter, randomized, double-blind, placebo-controlled studies (referred to as Studies 1 and 2) in women (18 to 45 years) with PPD who met the Diagnostic and Statistical Manual of Mental Disorders criteria for a major depressive episode (DSM-IV) with onset of symptoms in the third trimester or within 4 weeks of delivery. In these studies, patients received a 60-hour continuous intravenous infusion of ZULRESSO or placebo and were then followed for 4 weeks. Study 1 (NCT02942004) included patients with severe PPD (Hamilton Depression Rating Scale (HAM-D) score ≥26), and Study 2 (NCT02942017) included patients with moderate PPD (HAM-D score of 20 to 25). A titration to the recommended target dosage of 90 mcg/kg/hour was evaluated in both studies (patients received 30 mcg/kg/hour for 4 hours, 60 mcg/kg/hour for 20 hours, 90 mcg/kg/hour for 28 hours, followed by a taper to 60 mcg/kg/hour for 4 hours and then 30 mcg/kg/hour for 4 hours). A titration to a target dosage of 60 mcg/kg/hour (patients received 30 mcg/kg/hour for 4 hours, 60 mcg/kg/hour for 52 hours, then 30 mcg/kg/hour for 4 hours) was also evaluated in Study 1.
Demographic and baseline disease characteristics were generally similar across treatment groups in the pooled Studies 1 and 2. Most patients were White (63%) or Black (34%); 18% of patients identified as Hispanic or Latina; the average age of women receiving ZULRESSO was 28 years. Most patients (76%) had onset of PPD symptoms within 4 weeks after delivery, with the remainder having onset during the third trimester. Baseline oral antidepressant use was reported for 23% of patients.
The primary endpoint was the mean change from baseline in depressive symptoms as measured by the HAM-D total score at the end of the infusion (Hour 60). A pre-specified secondary efficacy endpoint was the mean change from baseline in HAM-D total score at Day 30. In both placebo-controlled studies, titration to a target dose of ZULRESSO 90 mcg/kg/hour was superior to placebo in improvement of depressive symptoms. In a group of 38 patients in Study 1, a ZULRESSO titration to a target dose of 60 mcg/kg/hour was also superior to placebo in improvement of depressive symptoms.
Table 3. Results for the Primary Endpoint – HAM-D Total Score (Studies 1 and 2):
| Study Number | Treatment Group (# ITT subject) | Primary Endpoint: Change from Baseline in HAM-D Total Score at Hour 60 | ||
|---|---|---|---|---|
| Mean Baseline Score (SD) | LS Mean Change from Baseline (SE) | Placebo-subtracted Difference (95% CI) Unadjusted p-value | ||
| 1 | ZULRESSO target dosage 90 mcg/kg/hour (n=41)* | 28.4 (2.5) | -17.7 (1.2) | -3.7 (-6.9, -0.5) P=0.0252 |
| Placebo (n=43) | 28.6 (2.5) | -14.0 (1.1) | ||
| ZULRESSO target dosage 60 mcg/kg/hour (n=38)* | 29.0 (2.7) | -19.5 (1.2) | -5.5 (-8.8, -2.2) P=0.0013 | |
| Placebo (n=43) | 28.6 (2.5) | -14.0 (1.1) | ||
| 2 | ZULRESSO target dosage 90 mcg/kg/hour (n=51)* | 22.6 (1.6) | -14.6 (0.8) | -2.5 (-4.5, -0.5) P=0.0160 |
| Placebo (n=53) | 22.7 (1.6) | -12.1 (0.8) | ||
HAM-D: Hamilton depression rating scale; ITT: intention to treat; SD: standard deviation; LS: least squares; SE: standard error; CI: confidence interval; *: statistically significant after multiplicity adjustments
Examination of subgroups by race did not suggest differences in response.
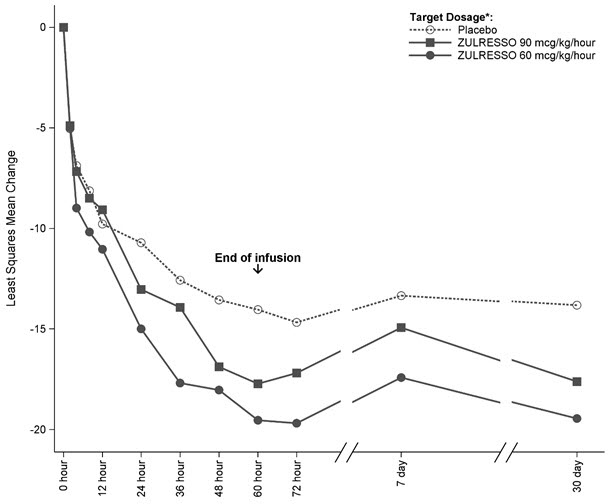
Time Course of Treatment Response
Figure 1 shows the time course of response for the ZULRESSO 90 mcg/kg/hour-target and 60 mcg/kg/hour-target groups compared to the placebo group for Study 1.
Figure 1. Change from Baseline in HAM-D Total Score Over Time (Days) in Study 1:
* ZULRESSO was administered via a 60-hour intravenous infusion as follows:
90 mcg/kg/hour-target dosage: 30 mcg/kg/hour for 4 hours, 60 mcg/kg/hour for 20 hours, 90 mcg/kg/hour for 28 hours, 60 mcg/kg/hour for 4 hours, 30 mcg/kg/hour for 4 hours
60 mcg/kg/hour-target dosage: 30 mcg/kg/hour for 4 hours, 60 mcg/kg/hour for 52 hours, 30 mcg/kg/hour for 4 hours
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.