ZYDELIG Film-coated tablet Ref.[9140] Active ingredients: Idelalisib
Source: European Medicines Agency (EU) Revision Year: 2020 Publisher: Gilead Sciences Ireland UC, Carrigtohill, County Cork, T45 DP77, Ireland
Pharmacodynamic properties
Pharmacotherapeutic group: antineoplastic agents, other antineoplastic agents
ATC code: L01XX47
Mechanism of action
Idelalisib inhibits phosphatidylinositol 3-kinase p110δ (PI3Kδ), which is hyperactive in B-cell malignancies and is central to multiple signalling pathways that drive proliferation, survival, homing, and retention of malignant cells in lymphoid tissues and bone marrow. Idelalisib is a selective inhibitor of adenosine-5'-triphosphate (ATP) binding to the catalytic domain of PI3Kδ, resulting in inhibition of the phosphorylation of the key lipid second messenger phosphatidylinositol and prevention of Akt (protein kinase B) phosphorylation.
Idelalisib induces apoptosis and inhibits proliferation in cell lines derived from malignant B-cells and in primary tumour cells. Through inhibition of chemokine receptors CXCR4 and CXCR5 signalling induced by the chemokines CXCL12 and CXCL13, respectively, idelalisib inhibits homing and retention of malignant B-cells in the tumour microenvironment including lymphoid tissues and the bone marrow.
No mechanistic explanations for the development of resistance to treatment with idelalisib have been identified from clinical studies. Further investigation of this topic in current B-cell malignancy studies is not planned.
Pharmacodynamic effects
Electrocardiographic
The effect of idelalisib (150 mg and 400 mg) on the QT/QTc interval was evaluated in a placebo- and positive-controlled (moxifloxacin 400 mg) crossover study in 40 healthy subjects. At a dose 2.7 times the maximum recommended dose, idelalisib did not prolong the QT/QTc interval (i.e., <10 ms).
Lymphocytosis
Upon initiation of idelalisib, a temporary increase in lymphocyte counts (i.e., ≥50% increase from baseline and above absolute lymphocyte count of 5,000/mcL) has been observed. This occurs in approximately two-thirds of patients with CLL treated with idelalisib monotherapy and one-fourth of patients with CLL treated with idelalisib combination therapy. The onset of isolated lymphocytosis typically occurs during the first 2 weeks of idelalisib therapy and is often associated with reduction of lymphadenopathy. This observed lymphocytosis is a pharmacodynamic effect and should not be considered progressive disease in the absence of other clinical findings.
Clinical efficacy in chronic lymphocytic leukaemia
Idelalisib in combination with rituximab
Study 312-0116 was a Phase 3, randomised, double-blind, placebo-controlled study in 220 subjects with previously treated CLL who required treatment but were not considered suitable for cytotoxic chemotherapy. Subjects were randomised 1:1 to receive 8 cycles of rituximab (first cycle at 375 mg/m² body surface area [BSA], subsequent cycles at 500 mg/m² BSA) in combination with either an oral placebo twice daily or with idelalisib 150 mg taken twice daily until disease progression or unacceptable toxicity.
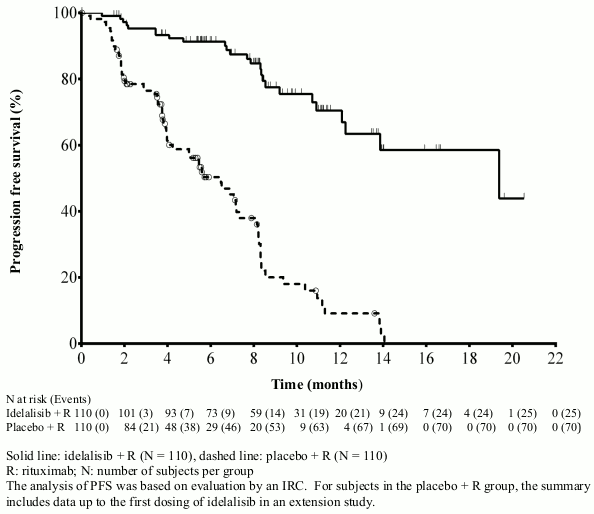
The median age was 71 years (range: 47 to 92) with 78.2% of subjects over 65 years; 65.5% were male, and 90.0% were white; 64.1% had a Rai stage of III or IV, and 55.9% had Binet Stage C. Most subjects had adverse cytogenetic prognostic factors: 43.2% had a 17p chromosomal deletion and/or tumour protein 53 (TP53) mutation, and 83.6% had unmutated genes for the immunoglobulin heavy chain variable region (IGHV). The median time from diagnosis of CLL to randomisation was 8.5 years. Subjects had a median Cumulative Illness Rating Scale (CIRS) score of 8. The median number of prior therapies was 3.0. Nearly all (95.9%) subjects had received prior anti-CD20 monoclonal antibodies. The primary endpoint was progression free survival (PFS). Efficacy results are summarised in Tables 3 and 4. The Kaplan-Meier curve for PFS is provided in Figure 1.
Compared with rituximab + placebo, treatment with idelalisib + rituximab resulted in statistically significant and clinically meaningful improvements in physical well-being, social well-being, functional well-being, as well as in the leukaemia-specific subscales of the Functional Assessment of Cancer Therapy: Leukaemia (FACT-LEU) instruments, and in statistically significant and clinically meaningful improvements in anxiety, depression and usual activities as measured by the EuroQoL Five-Dimensions (EQ-5D) instrument.
Table 3. Efficacy results from study 312-0116:
| Idelalisib + R N=110 | Placebo + R N=110 | |
|---|---|---|
| PFS | ||
| Median (months) (95% CI) | 19.4 (12.3–NR) | 6.5 (4.0–7.3) |
| Hazard ratio (95% CI) | 0.15 (0.09–0.24) | |
| P-value | p<0.0001 | |
| ORR* | ||
| n (%) (95% CI) | 92 (83.6%) (75.4–90.0) | 17 (15.5%) (9.3–23.6) |
| Odds ratio (95% CI) | 27.76 (13.40–57.49) | |
| P-value | p<0.0001 | |
| LNR** | ||
| n/N (%) (95% CI) | 102/106 (96.2%) (90.6-99.0) | 7/104 (6.7%) (2.7–13.4) |
| Odds ratio (95% CI) | 225.83 (65.56–777.94) | |
| P-value | p<0.0001 | |
| OS^ | ||
| Median (months) (95% CI) | NR (NR, NR) | 20.8 (14.8–NR) |
| Hazard ratio (95% CI) | 0.34 (0.19–0.60) | |
| P-value | 0.0001 | |
CI: confidence interval; R: rituximab; n: number of responding subjects; N: number of subjects per group; NR: not reached. The analyses of PFS, overall response rate (ORR) and lymph node response rate (LNR) were based on evaluation by an independent review committee (IRC).
* ORR defined as the proportion of subjects who achieved a complete response (CR) or partial response (PR) based on the 2013 National Comprehensive Cancer Network (NCCN) response criteria and Cheson (2012).
** LNR defined as the proportion of subjects who achieved a ≥50% decrease in the sum of products of the greatest perpendicular diameters of index lesions. Only subjects that had both baseline and ≥1 evaluable post-baseline assessments were included in this analysis.
^ Overall survival (OS) analysis includes data from subjects who received placebo + R on study 312-0116 and subsequently received idelalisib in an extension study, based on intent-to-treat analysis.
Table 4. Summary of PFS and response rates in pre-specified subgroups from study 312-0116:
| Idelalisib + R | Placebo + R | |
|---|---|---|
| 17p deletion/TP53 mutation | N=46 | N=49 |
| PFS median (months) (95% CI) | NR (12.3–NR) | 4.0 (3.7–5.7) |
| Hazard ratio (95% CI) | 0.13 (0.07–0.27) | |
| ORR (95% CI) | 84.8% (71.1–93.7) | 12.2% (4.6–24.8) |
| Unmutated IGHV | N=91 | N=93 |
| PFS median (months) (95% CI) | 19.4 (13.9–NR) | 5.6 (4.0–7.2) |
| Hazard ratio (95% CI) | 0.14 (0.08–0.23) | |
| ORR (95% CI) | 82.4% (73.0–89.6) | 15.1% (8.5–24.0) |
| Age ≥65 years | N=89 | N=83 |
| PFS median (months) (95% CI) | 19.4 (12.3–NR) | 5.7 (4.0–7.3) |
| Hazard ratio (95% CI) | 0.14 (0.08–0.25) | |
| ORR (95% CI) | 84.3% (75.0–91.1) | 16.9% (9.5–26.7) |
CI: confidence interval; R: rituximab; N: number of subjects per group; NR: not reached
Figure 1. Kaplan-Meier curve of PFS from study 312-0116 (intent-to-treat population):
Study 101-08/99 enrolled 64 subjects with previously untreated CLL, including 5 subjects with small lymphocytic lymphoma (SLL). Subjects received idelalisib 150 mg twice daily and rituximab 375 mg/m² BSA weekly for 8 doses. The ORR was 96.9%, with 12 CRs (18.8%) and 50 PRs (78.1%), including 3 CRs and 6 PRs in subjects with a 17p deletion and/or TP53 mutation and 2 CRs and 34 PRs in subjects with unmutated IGHV. The median duration of response (DOR) has not been reached.
Idelalisib in combination with ofatumumab
Study 312-0119 was a Phase 3, randomised, open-label, multicentre, parallel-group study in 261 subjects with previously treated CLL who had measurable lymphadenopathy, required treatment, and experienced CLL progression <24 months since the completion of the last prior therapy. Subjects were randomised 2:1 to receive idelalisib 150 mg twice daily and 12 infusions of ofatumumab over 24 weeks, or 12 infusions of ofatumumab only over 24 weeks. The first infusion of ofatumumab was administered at a dose of 300 mg and was continued at a dose of either 1,000 mg in the idelalisib + ofatumumab group or a dose of 2,000 mg in the ofatumumab only group, weekly for 7 doses, and then every 4 weeks for 4 doses. Idelalisib was taken until disease progression or unacceptable toxicity.
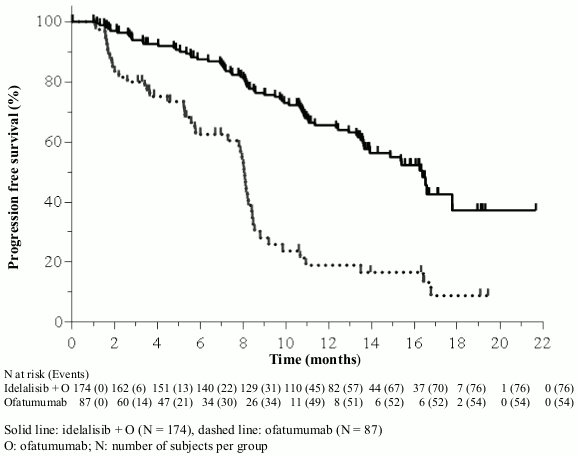
The median age was 68 years (range: 61 to 74) with 64.0% of subjects over 65 years; 71.3% were male, and 84.3% were white; 63.6% had a Rai stage of III or IV, and 58.2% had Binet Stage C. Most subjects had adverse cytogenetic prognostic factors: 39.5% had a 17p chromosomal deletion and/or TP53 mutation, and 78.5% had unmutated genes for IGHV. The median time since diagnosis was 7.7 years. Subjects had a median CIRS score of 4. The median number of prior therapies was 3.0. The primary endpoint was PFS. Efficacy results are summarised in Tables 5 and 6. The Kaplan-Meier curve for PFS is provided in Figure 2.
Table 5. Efficacy results from study 312-0119:
| Idelalisib + O N=174 | Ofatumumab N=87 | ||||
|---|---|---|---|---|---|
| PFS | Median (months) (95% CI) | 16.3 (13.6–17.8) | 8.0 (5.7–8.2) | ||
| Hazard ratio (95% CI) | 0.27 (0.19-0.39) | ||||
| P-value | <0.0001 | ||||
| ORR* | |||||
| n (%) (95% CI) | 131 (75.3%) (68.2–81.5) | 16 (18.4%) (10.9–28.1) | |||
| Odds ratio (95% CI) | 15.94 (7.8–32.58) | ||||
| P-value | <0.0001 | ||||
| LNR** | |||||
| n/N (%) (95% CI) | 153/164 (93.3%) (88.3–96.6) | 4/81 (4.9%) (1.4–12.2) | |||
| Odds ratio (95% CI) | 486.96 (97.91–2,424.85) | ||||
| P-value | <0.0001 | ||||
| OS | |||||
| Median (months) (95% CI) | 20.9 (20.9, NR) | 19.4 (16.9, NR) | |||
| Hazard ratio (95% CI) | 0.74 (0.44–1.25) | ||||
| P-value | 0.27 | ||||
CI: confidence interval; O: ofatumumab; n: number of responding subjects; N: number of subjects per group; NR: not reached. The analyses of PFS, overall response rate (ORR) and lymph node response rate (LNR) were based on evaluation by an independent review committee (IRC).
* ORR defined as the proportion of subjects who achieved a complete response (CR) or partial response (PR) and maintained their response for at least 8 weeks.
** LNR defined as the proportion of subjects who achieved a ≥50% decrease in the sum of products of the greatest perpendicular diameters of index lesions. Only subjects that had both baseline and ≥1 evaluable post-baseline assessments were included in this analysis.
Table 6. Summary of PFS and response rates in pre-specified subgroups from study 312-0119:
| Idelalisib + O | Ofatumumab | |
|---|---|---|
| 17p deletion/TP53 mutation | N=70 | N=33 |
| PFS median (months) (95% CI) | 13.7 (11.0–17.8) | 5.8 (4.5–8.4) |
| Hazard ratio (95% CI) | 0.32 (0.18–0.57) | |
| ORR (95% CI) | 72.9% (60.9–82.8) | 15.2% (5.1–31.9) |
| Unmutated IGHV | N=137 | N=68 |
| PFS median (months) (95% CI) | 14.9 (12.4–17.8) | 7.3 (5.3–8.1) |
| Hazard ratio (95% CI) | 0.25 (0.17–0.38) | |
| ORR (95% CI) | 74.5% (66.3–81.5) | 13.2% (6.2–23.6) |
| Age ≥65 years | N=107 | N=60 |
| PFS median (months) (95% CI) | 16.4 (13.4–17.8) | 8.0 (5.6–8.4) |
| Hazard ratio (95% CI) | 0.30 (0.19–0.47) | |
| ORR (95% CI) | 72.0% (62.5–80.2) | 18.3% (9.5–30.4) |
CI: confidence interval; O: ofatumumab; N: number of subjects per group
Figure 2. Kaplan-Meier curve of PFS from study 312-0119 (intent-to-treat population):
Clinical efficacy in follicular lymphoma
The safety and efficacy of idelalisib were assessed in a single-arm, multicentre clinical study (study 101-09) conducted in 125 subjects with indolent B-cell non-Hodgkin lymphoma (iNHL, including: FL, n=72; SLL, n=28; lymphoplasmacytic lymphoma/Waldenström macroglobulinaemia [LPL/WM], n=10; and marginal zone lymphoma [MZL], n=15). All subjects were refractory to rituximab and 124 of 125 subjects were refractory to at least one alkylating agent. One hundred and twelve (89.6%) subjects were refractory to their last regimen prior to study entry.
Of the 125 subjects enrolled, 80 (64%) were male, the median age was 64 years (range: 33 to 87), and 110 (89%) were white. Subjects received 150 mg of idelalisib orally twice daily until evidence of disease progression or unacceptable toxicity.
The primary endpoint was the ORR defined as the proportion of subjects who achieved a CR or PR (based on the Revised Response Criteria for Malignant Lymphoma [Cheson]), and, for subjects with Waldenström macroglobulinaemia, a minor response (MR) (based on the Response Assessment for Waldenström macroglobulinaemia [Owen]). DOR was a secondary endpoint and was defined as the time from the first documented response (CR, PR, or MR) to the first documentation of disease progression or death from any cause. Efficacy results are summarised in Table 7.
Table 7. Summary of efficacy in Study 101-09 (IRC assessment):
| Characteristic | Overall iNHL cohort (N=125) n (%) | FL subset (N=72) n (%) |
|---|---|---|
| ORR* | 72 (57.6%) | 40 (55.6%) |
| 95% CI | 48.4–66.4 | 43.4–67.3 |
| Response category*† | ||
| CR | 13 (10.4%) | 12 (16.7%) |
| PR | 58 (46.4%) | 28 (38.9%) |
| DOR (months) median (95% CI) | 12.5 (7.4, 22.4) | 11.8 (6.2, 26.9) |
| PFS (months) median (95% CI) | 11.1 (8.3, 14.0) | 11.0 (8.0, 14.0) |
| OS (months) median (95% CI) | 48.6 (33.9, 71.7) | 61.2 (38.1, NR) |
CI: confidence interval; n: number of responding subjects
NR: not reached
* Response as determined by an independent review committee (IRC) where ORR = complete response (CR) + partial response (PR) + minor response (MR) in subjects with WM.
† In the overall iNHL cohort, 1 subject (0.6%) with WM had the best overall response of MR
The median DOR for all subjects was 12.5 months (12.5 months for SLL subjects, and 11.8 months for FL, 20.4 months for LPL/WM and 18.4 months for MZL subjects). Among the 122 subjects with measurable lymph nodes at both baseline and post-baseline, 71 subjects (58.2%) achieved a ≥50% decrease from baseline in the sum of the products of the diameters (SPD) of index lesions. Of the 53 subjects who did not respond, 41 (32.8%) had stable disease 10 (8.0%) had progressive disease, and 2 (1.6%) were not evaluable. The median OS, including long-term follow-up for all 125 subjects, was 48.6 months. The median OS, including long-term follow-up for all FL subjects was 61.2 months.
Paediatric population
The European Medicines Agency has deferred the obligation to submit the results of studies with idelalisib in one or more subsets of the paediatric population in the treatment of mature B-cell neoplasms (see section 4.2 for information on paediatric use).
Pharmacokinetic properties
Absorption
Following oral administration of a single dose of idelalisib, peak plasma concentrations were observed 2 to 4 hours post-dose under fed conditions and after 0.5 to 1.5 hours under fasted conditions.
Following 150 mg twice daily administration of idelalisib, average (range) Cmax and AUC at steady-state were 1,953 (272; 3,905) ng/mL and 10,439 (2,349; 29,315) ng•h/mL for idelalisib and 4,039 (669; 10,897) ng/mL and 39,744 (6,002; 119,770) ng•h/mL for GS-563117, respectively. The plasma exposures (Cmax and AUC) of idelalisib are approximately dose proportional between 50 mg and 100 mg and less than dose proportional above 100 mg.
Effects of food
Relative to fasting conditions, administration of an early capsule formulation of idelalisib with a high-fat meal resulted in no change in Cmax and a 36% increase in mean AUCinf. Idelalisib can be administered without regard to food.
Distribution
Idelalisib is 93% to 94% bound to human plasma proteins at concentrations observed clinically. The mean blood-to-plasma concentration ratio was approximately 0.5. The apparent volume of distribution for idelalisib (mean) was approximately 96 L.
Biotransformation
Idelalisib is metabolised primarily via aldehyde oxidase, and to a lesser extent via CYP3A and UGT1A4. The primary and only circulating metabolite, GS-563117, is inactive against PI3Kδ.
Elimination
The terminal elimination half-life of idelalisib was 8.2 (range: 1.9; 37.2) hours and the apparent clearance of idelalisib was 14.9 (range: 5.1; 63.8) L/h following idelalisib 150 mg twice daily oral administration. Following a single 150 mg oral dose of [14C]-labelled idelalisib, approximately 78% and 15% was excreted in faeces and urine, respectively. Unchanged idelalisib accounted for 23% of total radioactivity recovered in urine over 48 hours and 12% of total radioactivity recovered in faeces over 144 hours.
In vitro interaction data
In vitro data indicated that idelalisib is not an inhibitor of the metabolising enzymes CYP1A2, CYP2B6, CYP2C9, CYP2C19, CYP2D6, CYP3A, or UGT1A1, or of the transporters OAT1, OAT3, or OCT2.
GS-563117 is not an inhibitor of the metabolising enzymes CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6 or UGT1A1, or of the transporters P-gp, BCRP, OATP1B1, OATP1B3, OAT1, OAT3, or OCT2.
Special populations
Gender and race
Population pharmacokinetic analyses indicated that gender and race had no clinically relevant effect on the exposures to idelalisib or GS-563117.
Elderly
Population pharmacokinetic analyses indicated that age had no clinically relevant effect on the exposures to idelalisib or GS-563117, including elderly subjects (65 years of age and older), compared to younger subjects.
Renal impairment
A study of pharmacokinetics and safety of idelalisib was performed in healthy subjects and subjects with severe renal impairment (estimated CrCl 15 to 29 mL/min). Following a single 150 mg dose, no clinically relevant changes in exposures to idelalisib or GS-563117 were observed in subjects with severe renal impairment compared to healthy subjects.
Hepatic impairment
A study of pharmacokinetics and safety of idelalisib was performed in healthy subjects and subjects with moderate (Child-Pugh Class B) or severe (Child-Pugh Class C) hepatic impairment. Following a single 150 mg dose, idelalisib AUC (total, i.e., bound plus unbound) was ~60% higher in moderate and severe impairment compared to matched controls. The idelalisib AUC (unbound), after accounting for differences in protein binding, was ~80% (1.8-fold) higher in moderate and ~152% (2.5-fold) higher in severe impairment compared to matched controls.
Paediatric population
The pharmacokinetics of idelalisib in paediatric subjects has not been established (see section 4.2).
Preclinical safety data
Repeated dose toxicity
Idelalisib induced lymphoid depletion in spleen, thymus, lymph nodes and gut-associated lymphoid tissue. In general, B-lymphocyte dependent areas were more affected than T-lymphocyte dependent areas. In rats, idelalisib has the potential to inhibit T-dependent antibody responses. However, idelalisib did not inhibit the normal host response to Staphylococcus aureus and did not exacerbate the myelosuppressive effect of cyclophosphamide. Idelalisib is not considered to have broad immunosuppressive activity.
Idelalisib induced inflammatory changes in both rats and dogs. In studies up to 4 weeks in rats and dogs, hepatic necrosis was observed at 7 and 5 times the human exposure based on AUC, respectively. Serum transaminase elevations correlated with hepatic necrosis in dogs, but were not observed in rats. No hepatic impairment or chronic transaminase elevations were observed in rats or dogs in studies of 13 weeks and longer duration.
Genotoxicity
Idelalisib did not induce mutations in the microbial mutagenesis (Ames) assay, was not clastogenic in the in vitro chromosome aberration assay using human peripheral blood lymphocytes, and was not genotoxic in the in vivo rat micronucleus study.
Carcinogenicity
The carcinogenicity potential of idelalisib was evaluated in a 26-week transgenic RasH2 mouse study and a 2-year rat study. Idelalisib was not carcinogenic at exposures up to 1.4/7.9-fold (male/female) in mice compared to the exposure in patients with haematologic malignancies administered the recommended dose of 150 mg twice daily. A dose-related increase in pancreatic islet cell tumors was observed at low incidence in male rats at exposures up to 0.4-fold compared to the human exposure at the recommended dose; a similar finding was not observed in female rats at 0.62-fold exposure margin.
Reproductive and developmental toxicity
In an embryo-foetal development study in rats, increased post-implantation loss, malformations (absence of caudal vertebrae and in some cases also of sacral vertebrae), skeletal variations and lower foetal body weights were observed. Malformations were observed at exposures from 12 times the human exposure based on AUC. Effects on embryo-foetal development were not investigated in a second species.
Degeneration of the seminiferous tubules in the testes was observed in 2- to 13-week repeated dose studies in dogs and rats, but not in studies of 26 weeks and longer duration. In a rat male fertility study, decreases in epididymides and testes weight were observed but no adverse effects on mating or fertility parameters, and no degeneration or loss in spermatogenesis were observed. Female fertility was not affected in rats.
Phototoxicity
Evaluation of the potential for phototoxicity in the embryonic murine fibroblast cell line BALB/c 3T3 was inconclusive for idelalisib due to cytotoxicity in the in vitro assay. The major metabolite, GS-563117, may enhance phototoxicity when cells are simultaneously exposed to UVA light. There is a potential risk that idelalisib, via its major metabolite, GS-563117, may cause photosensitivity in treated patients.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.