Edoxaban
Chemical formula: C₂₄H₃₀ClN₇O₄S Molecular mass: 548.06 g/mol PubChem compound: 10280735
Mechanism of action
Edoxaban is a highly selective, direct and reversible inhibitor of factor Xa, the serine protease located in the final common pathway of the coagulation cascade. Edoxaban inhibits free factor Xa, and prothrombinase activity. Inhibition of factor Xa in the coagulation cascade reduces thrombin generation, prolongs clotting time and reduces the risk of thrombus formation.
Pharmacodynamic properties
Pharmacodynamic effects
Edoxaban produces rapid onset of pharmacodynamic effects within 1-2 hours, which corresponds with peak edoxaban exposure (Cmax). The pharmacodynamic effects measured by anti-factor Xa assay are predictable and correlate with the dose and the concentration of edoxaban. As a result of FXa inhibition, edoxaban also prolongs clotting time in tests such as prothrombin time (PT), and activated partial thromboplastin time (aPTT). Changes observed in these clotting tests are expected at the therapeutic dose, however, these changes are small, subject to a high degree of variability, and not useful in monitoring the anticoagulation effect of edoxaban.
Effects of coagulation markers when switching from rivaroxaban, dabigatran, or apixaban to edoxaban
In clinical pharmacology studies, healthy subjects received rivaroxaban 20 mg once daily, dabigatran 150 mg twice daily, or apixaban 5 mg twice daily, followed by a single dose of edoxaban 60 mg on Day 4. The effect on prothrombin time (PT) and other coagulation biomarkers (e.g. anti-FXa, aPTT) was measured. Following the switch to edoxaban on Day 4 the PT was equivalent to Day 3 of rivaroxaban and apixaban. For dabigatran higher aPTT activity was observed after edoxaban administration with prior dabigatran treatment compared to that after treatment with edoxaban alone.
This is considered to be due to the carry-over effect of dabigatran treatment, however, this did not lead to a prolongation of bleeding time. Based on these data, when switching from these anticoagulants to edoxaban, the first dose of edoxaban can be initiated at the time of the next scheduled dose of the previous anticoagulant.
Pharmacokinetic properties
Absorption
Edoxaban is absorbed with peak plasma concentrations within 1-2 hours. The absolute bioavailability is approximately 62%. Food increases peak exposure to a varying extent, but has minimal effect on total exposure. Edoxaban was administered with or without food in the ENGAGE AF-TIMI 48 and the Hokusai-VTE studies. Edoxaban is poorly soluble at pH of 6.0 or higher. Co-administration of protonpump inhibitors had no relevant impact on edoxaban exposure.
Distribution
Disposition is biphasic. The volume of distribution is 107 (19.9) L mean (SD). In vitro plasma protein binding is approximately 55%. There is no clinically relevant accumulation of edoxaban (accumulation ratio 1.14) with once daily dosing. Steady state concentrations are achieved within 3 days.
Biotransformation
Unchanged edoxaban is the predominant form in plasma. Edoxaban is metabolised via hydrolysis (mediated by carboxylesterase 1), conjugation or oxidation by CYP3A4/5 (<10%). Edoxaban has three active metabolites, the predominant metabolite (M-4), formed by hydrolysis, is active and reaches less than 10% of the exposure of the parent compound in healthy subjects. Exposure to the other metabolites is less than 5%. Edoxaban is a substrate for the efflux transporter P-glycoprotein (Pgp), but not a substrate for uptake transporters such as organic anion transporter polypeptide OATP1B1, organic anion transporters OAT1 or OAT3 or organic cation transporter OCT2. Its active metabolite is a substrate for OATP1B1.
Elimination
In healthy subjects, the total clearance is estimated as 22 (±3) L/hour; 50% is renally cleared (11 L/hour). Renal clearance accounts for approximately 35% of the administered dose. Metabolism and biliary/intestinal excretion account for the remaining clearance. The t½ for oral administration is 10-14 hours.
Linearity/non-linearity
Edoxaban displays approximately dose-proportional pharmacokinetics for doses of 15 mg to 60 mg in healthy subjects.
Special populations
Elderly
After taking renal function and body weight into account, age had no additional clinically significant effect on edoxaban pharmacokinetics in a population pharmacokinetic analysis of the pivotal Phase 3 study in NVAF (ENGAGE AF-TIMI 48).
Gender
After accounting for body weight, gender had no additional clinically significant effect on edoxaban pharmacokinetics in a population pharmacokinetic analysis of the Phase 3 study in NVAF (ENGAGE AF-TIMI 48).
Ethnic origin
In a population pharmacokinetic analysis of the ENGAGE AF-TIMI 48 study, peak and total exposure in Asian patients and non-Asian patients were comparable.
Renal impairment
The plasma AUCs for subjects with mild (CrCL >50-80 mL/min), moderate (CrCL 30-50 mL/min) and severe (CrCL <30 mL/min but not undergoing dialysis) renal impairment were increased by 32%, 74%, and 72%, respectively, relative to subjects with normal renal function. In patients with renal impairment the metabolite profile changes and a higher quantity of active metabolites are formed. There is a linear correlation between edoxaban plasma concentration and anti-FXa activity regardless of renal function.
Subjects with ESRD undergoing peritoneal dialysis had 93% higher total exposure compared with healthy subjects.
Population PK modeling indicates that exposure approximately doubles in patients with severe renal impairment (CrCL 15–29 mL/min) relative to patients with normal renal function.
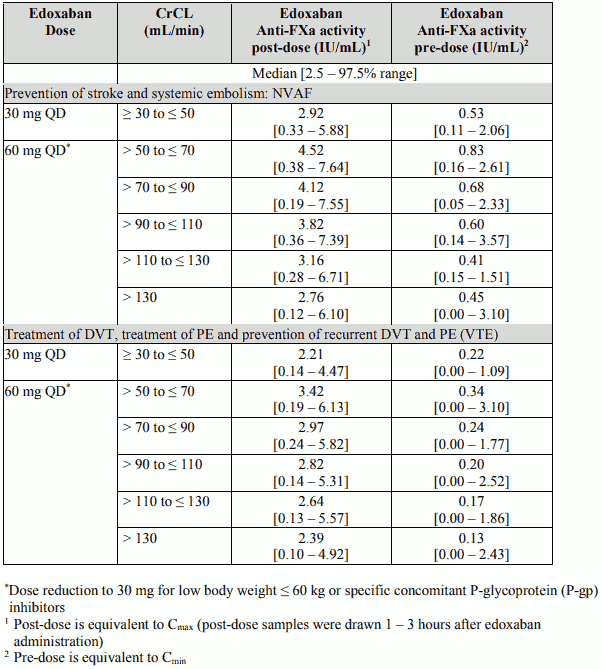
Anti-FXa activity by CrCL category
Table 12 below shows the edoxaban anti-Factor Xa activity by CrCL category for each indication.
Table 12: Edoxaban Anti-FXa activity by creatinine clearance
Although treatment with edoxaban does not require routine monitoring, the effect on anticoagulation can be estimated by a calibrated quantitative anti-Factor Xa assay which may be useful in exceptional situations where knowledge of edoxaban exposure may help to inform clinical decisions, e.g. overdose and emergency surgery.
Haemodialysis
A 4 hour haemodialysis session reduced total edoxaban exposures by less than 9%.
Hepatic impairment
Patients with mild or moderate hepatic impairment exhibited comparable pharmacokinetics and pharmacodynamics to their matched healthy control group. Edoxaban has not been studied in patients with severe hepatic impairment.
Body weight
In a population pharmacokinetic analysis of the ENGAGE AF-TIMI 48 study in NVAF, Cmax and AUC in patients with median low body weight (55 kg) were increased by 40% and 13%, respectively, as compared with patients with median high body weight (84 kg). In Phase 3 clinical studies (both NVAF and VTE indications) patients with body weight ≤60 kg had a 50% edoxaban dose reduction and had similar efficacy and less bleeding when compared to warfarin.
Pharmocokinetic/pharmacodynamic relationship(s)
PT, INR, aPTT and Anti-factor Xa correlate linearly with edoxaban concentrations.
Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity, carcinogenic potential, or phototoxicity.
Reproductive toxicology
Edoxaban showed vaginal haemorrhage at higher doses in rats and rabbits but had no effects in the reproductive performance of parent rats.
In rats, no effects on male or female fertility were seen.
In animal reproduction studies, rabbits showed increased incidence of gallbladder variations at a dosage of 200 mg/kg which is approximately 65 times the maximum recommended human dose (MRHD) of 60 mg/day based on total body surface area in mg/m². Increased post-implantation pregnancy losses occurred in rats at 300 mg/kg/day (approximately 49 times the MRHD) and in rabbits at 200 mg/kg/day (approximately 65 times the MRHD) respectively.
Edoxaban was excreted in the breast milk of lactating rats.
Environmental Risk Assessment (ERA)
The active substance edoxaban tosilate is persistent in the environment.
Related medicines
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.