Filgotinib
Chemical formula: C₂₁H₂₃N₅O₃S Molecular mass: 425.51 g/mol
Mechanism of action
Filgotinib is an adenosine triphosphate (ATP)-competitive and reversible inhibitor of the JAK family. JAKs are intracellular enzymes which transmit signals arising from cytokine or growth factor-receptor interactions on the cellular membrane. JAK1 is important in mediating inflammatory cytokine signals, JAK2 in mediating myelopoiesis and erythropoiesis and JAK3 plays critical roles in immune homeostasis and lymphopoiesis. Within the signalling pathway, JAKs phosphorylate and activate signal transducers and activators of transcription (STATs) which modulate intracellular activity including gene expression. Filgotinib modulates these signalling pathways by preventing the phosphorylation and activation of STATs.
In biochemical assays, filgotinib preferentially inhibited the activity of JAK1 and showed >5-fold higher potency of filgotinib for JAK1 over JAK2, JAK3 and TYK2. In human cellular assays, filgotinib preferentially inhibited JAK1/JAK3-mediated signalling downstream of the heterodimeric cytokine receptors for interleukin (IL)-2, IL-4 and IL-15, JAK1/2-mediated IL-6, and JAK1/TYK2-mediated type I interferons, with functional selectivity over cytokine receptors that signal via pairs of JAK2 or JAK2/TYK2. GS-829845, the primary metabolite of filgotinib, was approximately 10-fold less active than filgotinib in in vitro assays, while exhibiting a similar JAK1 preferential inhibitory activity. In an in vivo rat model, the overall pharmacodynamic effect was predominantly driven by the metabolite.
Pharmacodynamic properties
Pharmacodynamic effects
Inhibition of IL-6 induced STAT1 phosphorylation
Filgotinib administration resulted in a dose-dependent inhibition of IL-6 induced STAT1 phosphorylation in whole blood from healthy subjects. Filgotinib administration did not affect JAK2-associated GM-CSF induced STAT5 phosphorylation.
Immunoglobulins
In FINCH 1, 2, and 3, the median and interquartile ranges for serum IgG, IgM, and IgA values remained largely within the normal reference ranges through 24 weeks of treatment with filgotinib.
Haematologic effects
Treatment with filgotinib was associated with a small, transient increase in mean ALC that remained within normal reference ranges and gradually returned to at or near baseline levels with continued treatment by week 12. In FINCH 1, 2, and 3, median haemoglobin values remained stable within the normal range through 24 weeks of filgotinib treatment. A slight decrease in median platelet counts occurred within the first 4 weeks of filgotinib treatment and remained stable thereafter through 24 weeks. Median platelet counts remained within the normal range.
C-reactive protein
Decreases in serum C-reactive protein (CRP) were observed as early as 2 weeks after starting treatment with filgotinib and were maintained through 24 weeks of treatment.
Pharmacokinetic properties
Absorption
Following oral administration, filgotinib was absorbed quickly and its median peak plasma concentration was observed 2 to 3 hours postdose after multiple dosing; the median peak plasma concentrations of its primary metabolite GS-829845 were observed 5 hours postdose after multiple dosing. Filgotinib and GS-829845 exposures (AUC) and Cmax were similar in healthy adult subjects and patients with rheumatoid arthritis. Filgotinib and GS-829845 exposures (AUC) and Cmax are dose-proportional over the therapeutic dose range. Steady-state concentrations of filgotinib are achieved in 2-3 days with negligible accumulation after once daily administration. Steady-state concentrations of GS-829845 are achieved in 4 days with approximately 2-fold accumulation after once daily dosing of filgotinib.
There were no clinically relevant differences in exposures when filgotinib was administered with a high-fat or low-fat meal as compared to a fasted state. Filgotinib can be administered with or without food.
The multiple dose pharmacokinetic parameters of filgotinib and GS-829845 are provided in Table 1.
Table 1. Multiple dose pharmacokinetic parameters of filgotinib and GS-829845 following oral administration of filgotinib 200 mg with or without food in adults with moderate to severe active rheumatoid arthritis:
| Parametera Mean (%CV) | Filgotinibb | GS829845c |
|---|---|---|
| Cmax (µg/mL) | 2.15 (48.1) | 4.43 (29.3) |
| AUCtau (µg•h/mL) | 6.77 (43.7) | 83.2 (27.3) |
CV: coefficient of variation.
a From intensive PK analyses of studies FINCH 1, FINCH 2, and FINCH 3 in rheumatoid arthritis patients receiving 200 mg filgotinib once daily.
b N=37
c N=33
Distribution
Filgotinib and GS-829845 binding to human plasma proteins is low (55-59% and 39-44% bound, respectively). The blood-to-plasma ratio of filgotinib ranged from 0.85 to 1.1 indicating no preferential distribution of filgotinib and GS-829845 into blood cells. Filgotinib and GS-829845 are substrates of the P-gp transporter.
Biotransformation
Filgotinib is extensively metabolised with approximately 9.4% and 4.5% of an orally administered dose recovered as unchanged filgotinib in urine and faeces, respectively. Filgotinib is primarily metabolised by CES2, and to a lesser extent by CES1. Both CES2 and CES1 form GS-829845, an active circulating metabolite that is approximately 10-fold less potent than the parent compound. In a clinical pharmacology study, filgotinib and GS-829845 accounted for the majority of radioactivity circulating in plasma (2.9% and 92%, respectively). No other major metabolites were identified.
As both filgotinib and GS-829845 contribute to efficacy, their exposures were combined into a single parameter, AUCeff. AUCeff is the sum of the AUC of filgotinib and GS-829845, corrected for their respective molecular weights and potencies.
Elimination
Approximately 87% of the administered dose was eliminated in the urine as filgotinib and its metabolites, while about 15% of the dose was eliminated in the faeces. GS-829845 accounted for approximately 54% and 8.9% of dose recovered in urine and faeces, respectively. The mean terminal half-lives of filgotinib and GS-829845 were approximately 7 and 19 hours, respectively.
Other special populations
Weight, gender, race, and age
Bodyweight, gender, race, and age did not have a clinically relevant effect on the pharmacokinetics (AUC) of filgotinib or GS-829845.
Elderly
There were no clinically relevant differences in mean filgotinib and GS-829845 exposures (AUC and Cmax) between older patients aged ≥65 years relative to adult patients aged <65 years.
Renal impairment
The pharmacokinetics of filgotinib and GS-829845 were unaffected in subjects with mild renal impairment (CrCl 60 to <90 mL/min). Increases in exposures (AUC) of filgotinib, GS-829845, and combined AUCeff (≤2-fold), were observed in subjects with moderate renal impairment (CrCl 30 to <60 mL/min). In subjects with severe renal impairment (CrCl 15 to <30 mL/min), filgotinib exposure (AUC) increased by 2.2-fold and GS-829845 exposure significantly increased by 3.5-fold leading to a 3-fold increase in AUCeff. The pharmacokinetics of filgotinib has not been studied in subjects with end stage renal disease (CrCl <15 mL/min).
Hepatic impairment
No clinically relevant changes in the exposures (AUC) of filgotinib and GS-829845 individually, or their combined exposure (AUCeff), were observed in subjects with moderate hepatic impairment (Child-Pugh B). The pharmacokinetics of filgotinib has not been studied in subjects with severe hepatic impairment (Child-Pugh C).
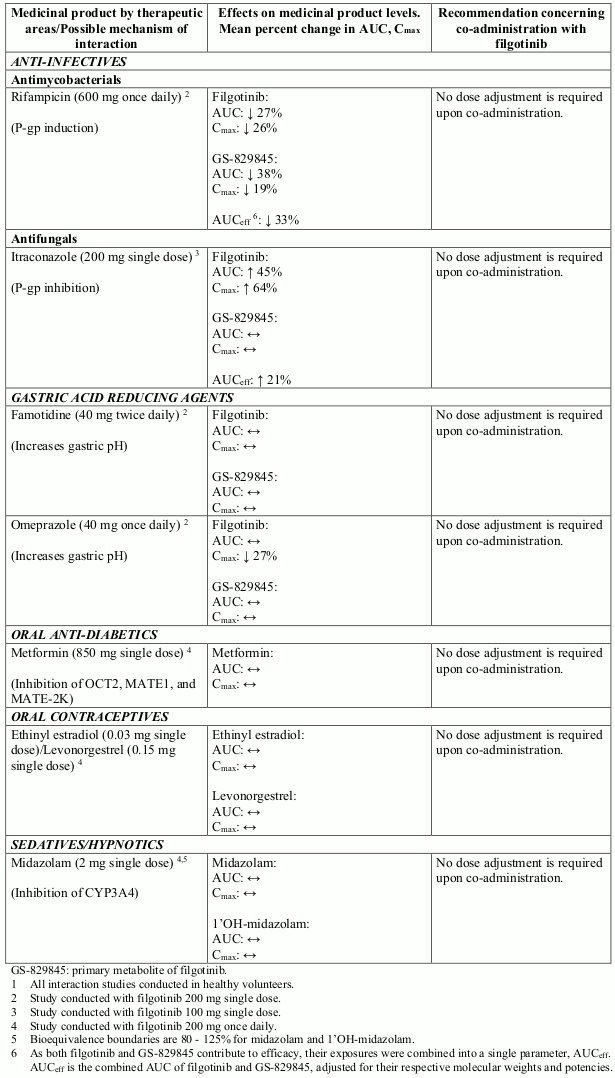
Effect of filgotinib on other medicinal products
Potential interactions between filgotinib and co-administered medicinal products are listed in Table 2 below (increase is indicated as “↑”, decrease as “↓”, and no change as “↔”; no effect boundaries are 70-143% unless otherwise indicated).
Table 2. Interaction studies with filgotinib1:
Potential for filgotinib to affect other medicinal products
In vitro data indicate that filgotinib and GS-829845 do not inhibit the activity of the following: CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, UGT1A1, UGT1A4, UGT1A6, UGT1A9, and UGT2B7 at clinically relevant concentrations. The potential for filgotinib to induce CYP2B6 constitutive androstane receptor (CAR) mediated metabolism in vivo is unknown. No conclusion can be drawn from the in vitro data regarding the potential of filgotinib to inhibit or induce CYP1A2. In vivo data demonstrated no inhibition or induction of CYP3A4 mediated metabolism.
In vitro studies indicate that filgotinib and GS-829845 are not inhibitors of OCT1, BSEP, OAT1, OAT3 or OAT4 at clinically relevant concentrations. In vitro data indicate that filgotinib and GS-829845 have the potential to inhibit OATP1B1, OATP1B3, OCT2, MATE1 (filgotinib only), and MATE-2K. While in vitro studies indicate that filgotinib is not an inhibitor of P-gp or BCRP, the results for GS-829845 are inconclusive and in vivo inhibition of P-gp or BCRP by GS-829845 cannot be excluded.
Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology.
The carcinogenic potential of filgotinib was evaluated in a 6-month rasH2 transgenic mouse study and a 2-year rat study. Filgotinib was not carcinogenic in mice at up to 150 mg/kg/day, which resulted in exposures of approximately 25 and 12 times the exposures in humans at the 100 mg and 200 mg once daily doses, respectively. In the 2-year rat study, filgotinib treatment resulted in an increase in incidence and decrease in latency of benign Leydig cell tumours at the highest dose of 45 mg/kg/day (exposures of approximately 4.2 times exposures in humans at the 200 mg once daily dose); the clinical relevance of this finding is low.
Filgotinib was not mutagenic or clastogenic in the in vitro bacterial reverse mutation assay, in vitro chromosome aberration assay, and in vivo rat micronucleus assay.
Adverse findings of degeneration/necrosis of incisor ameloblasts were observed in rats at exposures 21- to 28-fold greater than clinical exposures at the 200 mg filgotinib dose, with exposure margins at the NOAEL ranging from 3.5- to 8-fold. The human relevance of these dental findings is considered low since in contrast to adult patients, ameloblasts in rats persist into adulthood to support lifelong continuous incisor growth.
Impaired spermatogenesis and histopathological effects on male reproductive organs (testes and epididymis) were observed with filgotinib in rats and dogs. At the no-observed-adverse-effect-levels (NOAELs) in dogs (the most sensitive species), the exposure margin is 2.7-fold at the 200 mg once daily dose in humans. The severity of the histological effects was dose-dependent. Spermatogenic and histopathological effects were not fully reversible at lower exposures and were irreversible at exposure margins of approximately 7- to 9-fold the exposure at the 200 mg once daily dose in humans.
Embryo-foetal development studies in rats and rabbits demonstrated embryolethality and teratogenicity at exposures comparable to 200 mg filgotinib once daily dosing in humans. Visceral and skeletal malformations and/or variations were observed at all dose levels of filgotinib. Filgotinib was administered to pregnant rats at doses of 25, 50, and 100 mg/kg/day. Dose-related increases in the incidence of internal hydrocephaly, dilated ureters, and multiple vertebral anomalies were seen at all dose levels. At 100 mg/kg/day, an increased number of early and late resorptions were noted together with a decreased number of viable foetuses. In addition, foetal body weights were decreased.
In rabbits, filgotinib caused visceral malformations mainly in the lungs and cardiovascular system, at a dose level of 60 mg/kg/day. Filgotinib caused skeletal malformations affecting the vertebral column region at dose levels of 25 and 60 mg/kg/day, mainly in vertebra, ribs and sternebrae. Fused sternebrae also occurred at 10 mg/kg/day filgotinib. Retarded skeletal ossification was evidenced at 60 mg/kg/day.
No adverse effects on pre-/postnatal development were observed in rats in a pre- and postnatal development study of filgotinib and GS-829845. Filgotinib and GS-829845 were detected in nursing rat pups after administration of filgotinib to lactating female rats from gestation day 6 through 10 days post-partum at dose levels of 2, 5, and 15 mg/kg/day, likely due to the presence of filgotinib in milk. At the highest tested dose, maternal systemic exposure (AUC) to filgotinib in rats was approximately 2 times the exposure in humans at the 200 mg once daily dose; exposures in nursing pups were less than 6% that of maternal exposure on day 10 post-partum. Due to the low exposure of the animals, the pre-/postnatal development study was considered inconclusive.
Related medicines
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.