Tofacitinib
Chemical formula: C₁₆H₂₀N₆O Molecular mass: 312.37 g/mol PubChem compound: 9926791
Interactions
Tofacitinib interacts in the following cases:
Live vaccines
Prior to initiating tofacitinib, it is recommended that all patients be brought up to date with all immunisations in agreement with current immunisation guidelines. It is recommended that live vaccines not be given concurrently with tofacitinib. The decision to use live vaccines prior to tofacitinib treatment should take into account the pre-existing immunosuppression in a given patient.
Prophylactic zoster vaccination should be considered in accordance with vaccination guidelines. Particular consideration should be given to patients with longstanding RA who have previously received two or more biological DMARDs. If live zoster vaccine is administered; it should only be administered to patients with a known history of chickenpox or those that are seropositive for varicella zoster virus (VZV). If the history of chickenpox is considered doubtful or unreliable it is recommended to test for antibodies against VZV.
Vaccination with live vaccines should occur at least 2 weeks but preferably 4 weeks prior to initiation of tofacitinib or in accordance with current vaccination guidelines regarding immunomodulatory medicinal products. No data are available on the secondary transmission of infection by live vaccines to patients receiving tofacitinib.
CYP3A4 inducers
Since tofacitinib is metabolised by CYP3A4, interaction with medicinal products that inhibit or induce CYP3A4 is likely. Tofacitinib exposure is decreased when coadministered with potent CYP inducers (e.g. rifampicin).
CYP3A4 inhibitors, CYP2C19 inhibitors
Since tofacitinib is metabolised by CYP3A4, interaction with medicinal products that inhibit or induce CYP3A4 is likely. Tofacitinib exposure is increased when coadministered with potent inhibitors of CYP3A4 (e.g. ketoconazole) or when administration of one or more concomitant medicinal products results in both moderate inhibition of CYP3A4 and potent inhibition of CYP2C19 (e.g. fluconazole).
Tofacitinib exposure is decreased when coadministered with potent CYP inducers (e.g. rifampicin). Inhibitors of CYP2C19 alone or P-glycoprotein are unlikely to significantly alter the PK of tofacitinib.
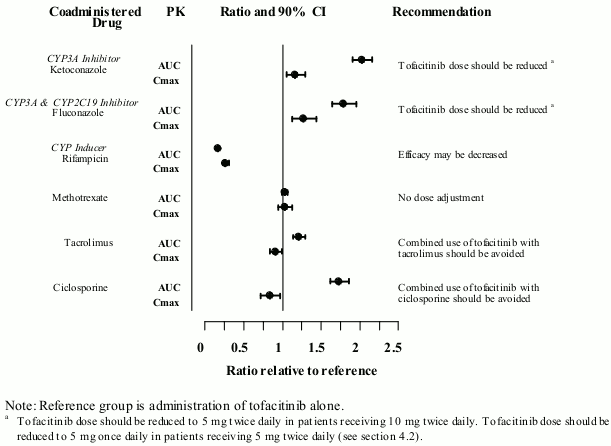
Coadministration with ketoconazole (strong CYP3A4 inhibitor), fluconazole (moderate CYP3A4 and potent CYP2C19 inhibitor), tacrolimus (mild CYP3A4 inhibitor) and c iclosporine (moderate CYP3A4 inhibitor) increased tofacitinib AUC, while rifampicin (potent CYP inducer) decreased tofacitinib AUC. Coadministration of tofacitinib with potent CYP inducers (e.g. rifampicin) may result in a loss of or reduced clinical response (see Figure 1). Coadministration of potent inducers of CYP3A4 with tofacitinib is not recommended. Coadministration with ketoconazole and fluconazole increased tofacitinib Cmax, while tacrolimus, ciclosporine and rifampicin decreased tofacitinib Cmax. Concomitant administration with MTX 15-25 mg once weekly had no effect on the PK of tofacitinib in RA patients (see Figure 1).
Figure 1. Impact of other medicinal products on PK of tofacitinib:
Hepatic impairment
Dose adjustment for hepatic impairment:
| Hepatic impairment category | Classification | Dose adjustment in hepatic impairment for different strength tablets |
|---|---|---|
| Mild | Child Pugh A | No dose adjustment required. |
| Moderate | Child Pugh B | Dose should be reduced to 5 mg once daily when the indicated dose in the presence of normal hepatic function is 5 mg twice daily. |
| Dose should be reduced to 5 mg twice daily when the indicated dose in the presence of normal hepatic function is 10 mg twice daily | ||
| Severe | Child Pugh C | Tofacitinib should not be used in patients with severe hepatic impairment |
Treatment with tofacitinib was associated with an increased incidence of liver enzyme elevation in some patients. Caution should be exercised when considering initiation of tofacitinib treatment in patients with elevated alanine aminotransferase (ALT) or aspartate aminotransferase (AST), particularly when initiated in combination with potentially hepatotoxic medicinal products such as MTX.
Following initiation, routine monitoring of liver tests and prompt investigation of the causes of any observed liver enzyme elevations are recommended to identify potential cases of drug-induced liver injury. If drug-induced liver injury is suspected, the administration of tofacitinib should be interrupted until this diagnosis has been excluded.
Renal impairment
Dose adjustment for renal impairment:
| Renal impairment category | Creatinine clearance | Dose adjustment in renal impairment for different strength tablets |
|---|---|---|
| Mild | 50-80 mL/min | No dose adjustment required. |
| Moderate | 30-49 mL/min | No dose adjustment required. |
| Severe (including patients undergoing haemodialysis) | <30 mL/min | Dose should be reduced to 5 mg once daily when the indicated dose in the presence of normal renal function is 5 mg twice daily. |
| Dose should be reduced to 5 mg twice daily when the indicated dose in the presence of normal renal function is 10 mg twice daily. | ||
| Patients with severe renal impairment should remain on a reduced dose even after haemodialysis |
Fertility
Formal studies of the potential effect on human fertility have not been conducted. Tofacitinib impaired female fertility but not male fertility in rats.
Ketoconazole, fluconazole, rifampicin, tacrolimus, ciclosporin
Impact of other medicinal products on PK of tofacitinib:
Increased lipid
Treatment with tofacitinib was associated with increases in lipid parameters such as total cholesterol, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol. Maximum effects were generally observed within 6 weeks. Assessment of lipid parameters should be performed after 8 weeks following initiation of tofacitinib therapy. Patients should be managed according to clinical guidelines for the management of hyperlipidaemia. Increases in total and LDL cholesterol associated with tofacitinib may be decreased to pretreatment levels with statin therapy.
Interstitial lung disease
Caution is also recommended in patients with a history of chronic lung disease as they may be more prone to infections. Events of interstitial lung disease (some of which had a fatal outcome) have been reported in patients treated with tofacitinib in RA clinical trials and in the post-marketing setting although the role of Janus kinase (JAK) inhibition in these events is not known. Asian RA patients are known to be at higher risk of interstitial lung disease, thus caution should be exercised in treating these patients.
Angioedema, urticaria
In post-marketing experience, cases of drug hypersensitivity associated with tofacitinib administration have been reported. Allergic reactions included angioedema and urticaria; serious reactions have occurred. If any serious allergic or anaphylactic reaction occurs, tofacitinib should be discontinued immediately.
Gastrointestinal perforation
Events of gastrointestinal perforation have been reported in clinical trials although the role of JAK inhibition in these events is not known. Tofacitinib should be used with caution in patients who may be at increased risk for gastrointestinal perforation (e.g. patients with a history of diverticulitis, patients with concomitant use of corticosteroids and/or nonsteroidal anti-inflammatory drugs). Patients presenting with new onset abdominal signs and symptoms should be evaluated promptly for early identification of gastrointestinal perforation.
Lymphopenia, neutropenia, anaemia
Tofacitinib treatment should be interrupted if a patient develops a serious infection until the infection is controlled.
Interruption of dosing may be needed for management of dose-related laboratory abnormalities including lymphopenia, neutropenia, and anaemia. As described in tables below, recommendations for temporary dose interruption or permanent discontinuation of treatment are made according to the severity of laboratory abnormalities.
It is recommended not to initiate dosing in patients with an absolute lymphocyte count (ALC) less than 750 cells/mm³.
Low absolute lymphocyte count:
| Low absolute lymphocyte count (ALC) | |
|---|---|
| Lab value (cells/mm³) | Recommendation |
| ALC greater than or equal to 750 | Dose should be maintained. |
| ALC 500-750 | For persistent (2 sequential values in this range on routine testing) decrease in this range, dosing should be reduced or interrupted until ALC is greater than 750. |
| For patients receiving tofacitinib 10 mg twice daily, dosing should be reduced to tofacitinib 5 mg twice daily. | |
| For patients receiving tofacitinib 5 mg twice daily, dosing should be interrupted. | |
| When ALC is greater than 750, treatment should be resumed as clinically appropriate | |
| ALC less than 500 | If lab value confirmed by repeat testing within 7 days, dosing should be discontinued. |
It is recommended not to initiate dosing in patients with an absolute neutrophil count (ANC) less than 1,000 cells/mm³.
Low absolute neutrophil count:
| Low absolute neutrophil count (ANC) | |
|---|---|
| Lab Value (cells/mm³) | Recommendation |
| ANC greater than 1,000 | Dose should be maintained. |
| ANC 500–1,000 | For persistent (2 sequential values in this range on routine testing) decreases in this range, dosing should be reduced or interrupted until ANC is greater than 1,000. |
| For patients receiving tofacitinib 10 mg twice daily, dosing should be reduced to tofacitinib 5 mg twice daily. | |
| For patients receiving tofacitinib 5 mg twice daily, dosing should be interrupted. | |
| When ANC is greater than 1,000, treatment should be resumed as clinically appropriate | |
| ANC less than 500 | If lab value confirmed by repeat testing within 7 days, dosing should be discontinued. |
It is recommended not to initiate dosing in patients with haemoglobin less than 9 g/dL.
Low haemoglobin value:
| Low haemoglobin value | |
|---|---|
| Lab value (g/dL) | Recommendation |
| Less than or equal to 2 g/dL decrease and greater than or equal to 9.0 g/dL | Dose should be maintained. |
| Greater than 2 g/dL decrease or less than 8.0 g/dL (confirmed by repeat testing) | Dosing should be interrupted until haemoglobin values have normalised. |
Pregnancy
There are no adequate and well-controlled studies on the use of tofacitinib in pregnant women. Tofacitinib has been shown to be teratogenic in rats and rabbits, and to affect parturition and peri/postnatal development.
As a precautionary measure, the use of tofacitinib during pregnancy is contraindicated.
Nursing mothers
It is not known whether tofacitinib is secreted in human milk. A risk to the breast-fed child cannot be excluded. Tofacitinib was secreted in the milk of lactating rats. As a precautionary measure, the use of tofacitinib during breast-feeding is contraindicated.
Carcinogenesis, mutagenesis and fertility
Women of childbearing potential/contraception in females
Women of childbearing potential should be advised to use effective contraception during treatment with tofacitinib and for at least 4 weeks after the last dose.
Fertility
Formal studies of the potential effect on human fertility have not been conducted. Tofacitinib impaired female fertility but not male fertility in rats.
Effects on ability to drive and use machines
Tofacitinib has no or negligible influence on the ability to drive and use machines.
Adverse reactions
Summary of the safety profile
Rheumatoid arthritis
The most common serious adverse reactions were serious infections. The most common serious infections reported with tofacitinib were pneumonia, cellulitis, herpes zoster, urinary tract infection, diverticulitis, and appendicitis. Among opportunistic infections, TB and other mycobacterial infections, cryptococcus, histoplasmosis, oesophageal candidiasis, multidermatomal herpes zoster, cytomegalovirus, BK virus infections and listeriosis were reported with tofacitinib. Some patients have presented with disseminated rather than localised disease. Other serious infections that were not reported in clinical studies may also occur (e.g. coccidioidomycosis).
The most commonly reported adverse reactions during the first 3 months in controlled clinical trials were headache, upper respiratory tract infections, nasopharyngitis, diarrhoea, nausea and hypertension.
The proportion of patients who discontinued treatment due to adverse reactions during first 3 months of the double-blind, placebo or MTX controlled studies was 3.8% for patients taking tofacitinib. The most common infections resulting in discontinuation of therapy were herpes zoster and pneumonia.
Psoriatic arthritis
Overall, the safety profile observed in patients with active PsA treated with tofacitinib was consistent with the safety profile observed in patients with RA treated with tofacitinib.
Ulcerative colitis
The most commonly reported adverse reactions in patients receiving tofacitinib 10 mg twice daily in the induction studies were headache, nasopharyngitis, nausea, and arthralgia.
In the induction and maintenance studies, across tofacitinib and placebo treatment groups, the most common categories of serious adverse reactions were gastrointestinal disorders and infections, and the most common serious adverse reaction was worsening of UC.
Overall, the safety profile observed in patients with UC treated with tofacitinib was consistent with the safety profile of tofacitinib in the RA indication.
List of adverse reactions
The ADRs listed below are from clinical studies in patients with RA, PsA, and UC and are presented by System Organ Class (SOC) and frequency categories, defined using the following convention: very common (≥1/10), common (≥1/100 to <1/10), uncommon (≥1/1,000 to <1/100), rare (≥1/10,000 to <1/1,000), very rare (<1/10,000), or not known (cannot be estimated from the available data). Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness.
Infections and infestations
Common: Pneumonia, Influenza, Herpes zoster, Urinary tract infection, Sinusitis, Bronchitis, Nasopharyngitis, Pharyngitis
Uncommon: Tuberculosis, Diverticulitis, Pyelonephritis, Cellulitis, Herpes simplex, Gastroenteritis viral, Viral infection
Rare: Sepsis, Urosepsis, Disseminated TB, Necrotizing fasciitis, Bacteraemia, Staphylococcal bacteraemia, Pneumocystis jirovecii pneumonia, Pneumonia pneumococcal, Pneumonia bacterial, Encephalitis, Atypical mycobacterial infection, Cytomegalovirus infection, Arthritis bacterial
Very rare: Tuberculosis of central nervous system, Meningitis cryptococcal, Mycobacterium avium complex infection
Neoplasms benign, malignant and unspecified (incl cysts and polyps)
Uncommon: Non-melanoma skin cancers
Blood and lymphatic system disorders
Common: Anaemia
Uncommon: Leukopenia, Lymphopenia, Neutropenia
Immune system disorders
Not known: Drug hypersensitivity*, Angioedema*, Urticaria*
Metabolism and nutrition disorders
Uncommon: Dyslipidaemia, Hyperlipidaemia, Dehydration
Psychiatric disorders
Uncommon: Insomnia
Nervous system disorders
Common: Headache
Uncommon: Paraesthesia
Vascular disorders
Common: Hypertension
Uncommon: Venous thromboembolism**
Respiratory, thoracic and mediastinal disorders
Common: Cough
Uncommon: Dyspnoea, Sinus congestion
Gastrointestinal disorders
Common: Abdominal pain, Vomiting, Diarrhoea, Nausea, Gastritis, Dyspepsia
Hepatobiliary disorders
Uncommon: Hepatic steatosis, Hepatic enzyme increased, Transaminases increased, Liver function test abnormal, Gamma glutamyltransferase increased
Skin and subcutaneous tissue disorders
Common: Rash
Uncommon: Erythema, Pruritus
Musculoskeletal and connective tissue disorders
Common: Arthralgia
Uncommon: Musculoskeletal pain, Joint swelling, Tendonitis
General disorders and administration site conditions
Common: Pyrexia, Oedema peripheral, Fatigue
Investigations
Common: Blood creatine phosphokinase increased
Uncommon: Blood creatinine increased, Blood cholesterol increased, Low density lipoprotein increased, Weight increased
Injury, poisoning and procedural complications
Uncommon: Ligament sprain, Muscle strain
* Spontaneous reporting data
** Venous thromboembolism includes PE and DVT
Description of selected adverse reactions
Venous thromboembolism
Rheumatoid arthritis
In a large, randomised post-authorisation safety surveillance study of rheumatoid arthritis patients who were 50 years of age and older and had at least onecardiovascular (CV) risk factor, VTE was observed at an increased and dose-dependent incidence in patients treated with tofacitinib compared to TNF inhibitors. The majority of these events were serious and some resulted in death. The incidence rates (95% CI) for PE for tofacitinib 10 mg twice daily, tofacitinib 5 mg twice daily, and TNF inhibitors were 0.54 (0.32-0.87), 0.27 (0.12-0.52), and 0.09 (0.02-0.26) patients with events per 100 patient-years, respectively. Compared with TNF inhibitors, the hazard ratio (HR) for PE was 5.96 (1.75-20.33) and 2.99 (0.81-11.06) for tofacitinib 10 mg twice daily and tofacitinib 5 mg twice daily, respectively.
In a subgroup analysis in patients with VTE risk factors in the above-mentioned study, the risk for PE was further increased. Compared with TNF inhibitors, the HR for PE was 9.14 (2.11-39.56) for tofacitinib 10 mg twice daily and 3.92 (0.83-18.48) for tofacitinib 5 mg twice daily.
Ulcerative colitis (UC)
In the UC ongoing extension trial, cases of PE and DVT have been observed in patients using tofacitinib 10 mg twice daily and with underlying VTE risk factor(s).
Overall infections
Rheumatoid arthritis
In controlled phase 3 clinical studies, the rates of infections over 0-3 months in the 5 mg twice daily (total 616 patients) and 10 mg twice daily (total 642 patients) tofacitinib monotherapy groups were 16.2% (100 patients) and 17.9% (115 patients), respectively, compared to 18.9% (23 patients) in the placebo group (total 122 patients). In controlled phase 3 clinical studies with background DMARDs, the rates of infections over 0-3 months in the 5 mg twice daily (total 973 patients) and 10 mg twice daily (total 969 patients) tofacitinib plus DMARD group were 21.3% (207 patients) and 21.8% (211 patients), respectively, compared to 18.4% (103 patients) in the placebo plus DMARD group (total 559 patients).
The most commonly reported infections were upper respiratory tract infections and nasopharyngitis (3.7% and 3.2%, respectively).
The overall incidence rate of infections with tofacitinib in the long-term safety all exposure population (total 4,867 patients) was 46.1 patients with events per 100 patient-years (43.8 and 47.2 patients with events for 5 mg and 10 mg twice daily, respectively). For patients (total 1,750) on monotherapy, the rates were 48.9 and 41.9 patients with events per 100 patient-years for 5 mg and 10 mg twice daily, respectively. For patients (total 3,117) on background DMARDs, the rates were 41.0 and 50.3 patients with events per 100 patient-years for 5 mg and 10 mg twice daily, respectively.
Ulcerative colitis
In the randomised 8-week Phase ⅔ induction studies, the proportions of patients with infections were 21.1% (198 patients) in the tofacitinib 10 mg twice daily group compared to 15.2% (43 patients) in the placebo group. In the randomised 52-week phase 3 maintenance study, the proportion of patients with infections were 35.9% (71 patients) in the 5 mg twice daily and 39.8% (78 patients) in the 10 mg twice daily tofacitinib groups, compared to 24.2% (48 patients) in the placebo group.
In the entire treatment experience with tofacitinib, the most commonly reported infection was nasopharyngitis, occurring in 18.2% of patients (211 patients).
In the entire treatment experience with tofacitinib, the overall incidence rate of infections was 60.3 events per 100 patient-years (involving 49.4% of patients; total 572 patients).
Serious infections
Rheumatoid arthritis
In the 6-month and 24-month, controlled clinical studies, the rate of serious infections in the 5 mg twice daily tofacitinib monotherapy group was 1.7 patients with events per 100 patient-years. In the 10 mg twice daily tofacitinib monotherapy group the rate was 1.6 patients with events per 100 patient-years, the rate was 0 events per 100 patient-years for the placebo group, and the rate was 1.9 patients with events per 100 patient-years for the MTX group.
In studies of 6-, 12-, or 24-month duration, the rates of serious infections in the 5 mg twice daily and 10 mg twice daily tofacitinib plus DMARD groups were 3.6 and 3.4 patients with events per 100 patient-years, respectively, compared to 1.7 patients with events per 100 patient-years in the placebo plus DMARD group.
In the long-term safety all exposure population, the overall rates of serious infections were 2.4 and 3.0 patients with events per 100 patient-years for 5 mg and 10 mg twice daily tofacitinib groups, respectively. The most common serious infections included pneumonia, herpes zoster, urinary tract infection, cellulitis, gastroenteritis and diverticulitis. Cases of opportunistic infections have been reported.
Ulcerative colitis
The incidence rates and types of serious infections in the UC clinical studies were generally similar to those reported in RA clinical studies with tofacitinib monotherapy treatment groups.
Serious infections in the elderly
Of the 4,271 patients who enrolled in RA studies I-VI, a total of 608 RA patients were 65 years of age and older, including 85 patients 75 years and older. The frequency of serious infection among tofacitinib-treated patients 65 years of age and older was higher than those under the age of 65 (4.8 per 100 patient-years versus 2.4 per 100 patient-years, respectively).
As there is a higher incidence of infections in the elderly population in general, caution should be used when treating the elderly.
Viral reactivation
Patients treated with tofacitinib who are Japanese or Korean, or patients with long standing RA who have previously received two or more biological DMARDs, or patients with an ALC less than 1,000 cells/mm³, or patients treated with 10 mg twice daily may have an increased risk of herpes zoster.
Laboratory tests
Lymphocytes
In the controlled RA clinical studies, confirmed decreases in ALC below 500 cells/mm³ occurred in 0.3% of patients and for ALC between 500 and 750 cells/mm³ in 1.9% of patients for the 5 mg twice daily and 10 mg twice daily doses combined.
In the RA long-term safety population, confirmed decreases in ALC below 500 cells/mm 3 occurred in 1.3% of patients and for ALC between 500 and 750 cells/mm³ in 8.4% of patients for the 5 mg twice daily and 10 mg twice daily doses combined.
Confirmed ALC less than 750 cells/mm³ were associated with an increased incidence of serious infections.
In the clinical studies in UC, changes in ALC observed with tofacitinib treatment were similar to the changes observed in clinical studies in RA.
Neutrophils
In the controlled RA clinical studies, confirmed decreases in ANC below 1,000 cells/mm³ occurred in 0.08% of patients for the 5 mg twice daily and 10 mg twice daily doses combined. There were no confirmed decreases in ANC below 500 cells/mm³ observed in any treatment group. There was no clear relationship between neutropenia and the occurrence of serious infections.
In the RA long-term safety population, the pattern and incidence of confirmed decreases in ANC remained consistent with what was seen in the controlled clinical studies.
In the clinical studies in UC, changes in ANC observed with tofacitinib treatment were similar to the changes observed in clinical studies in RA.
Liver enzyme tests
Confirmed increases in liver enzymes greater than 3 times the upper limit of normal (3 x ULN) were uncommonly observed in RA patients. In those patients experiencing liver enzyme elevation, modification of treatment regimen, such as reduction in the dose of concomitant DMARD, interruption of tofacitinib, or reduction in tofacitinib dose, resulted in decrease or normalisation of liver enzymes.
In the controlled portion of the RA phase 3 monotherapy study (0-3 months), ALT elevations greater than 3 x ULN were observed in 1.65%, 0.41%, and 0% of patients receiving placebo, tofacitinib 5 mg and 10 mg twice daily, respectively. In this study, AST elevations greater than 3 x ULN were observed in 1.65%, 0.41% and 0% of patients receiving placebo, tofacitinib 5 mg and 10 mg twice daily, respectively.
In the RA phase 3 monotherapy study (0-24 months), ALT elevations greater than 3 x ULN were observed in 7.1%, 3.0%, and 3.0% of patients receiving MTX, tofacitinib 5 mg and 10 mg twice daily, respectively. In this study, AST elevations greater than 3 x ULN were observed in 3.3%, 1.6% and 1.5% of patients receiving MTX, tofacitinib 5 mg and 10 mg twice daily, respectively.
In the controlled portion of the RA phase 3 studies on background DMARDs (0-3 months), ALT elevations greater than 3 x ULN were observed in 0.9%, 1.24% and 1.14% of patients receiving placebo, tofacitinib 5 mg and 10 mg twice daily, respectively. In these studies, AST elevations greater than 3 x ULN were observed in 0.72%, 0.5% and 0.31% of patients receiving placebo, tofacitinib 5 mg and 10 mg twice daily, respectively.
In the RA long-term extension studies, on monotherapy, ALT elevations greater than 3 x ULN were observed in 1.1% and 1.4% of patients receiving tofacitinib 5 mg and 10 mg twice daily, respectively. AST elevations greater than 3 x ULN were observed in <1.0% in both the tofacitinib 5 mg and 10 mg twice daily groups.
In the RA long-term extension studies, on background DMARDs, ALT elevations greater than 3 x ULN were observed in 1.8% and 1.6% of patients receiving tofacitinib 5 mg and 10 mg twice daily, respectively. AST elevations greater than 3 x ULN were observed in <1.0% in both the tofacitinib 5 mg and 10 mg twice daily groups.
In the clinical studies in UC, changes in liver enzyme tests observed with tofacitinib treatment were similar to the changes observed in clinical studies in RA.
Lipids
Elevations in lipid parameters (total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides) were first assessed at 1 month following initiation of tofacitinib in the controlled double-blind clinical trials of RA. Increases were observed at this time point and remained stable thereafter.
Changes in lipid parameters from baseline through the end of the study (6-24 months) in the controlled clinical studies in RA are summarised below:
- Mean LDL cholesterol increased by 15% in the tofacitinib 5 mg twice daily arm and 20% in the tofacitinib 10 mg twice daily arm at month 12, and increased by 16% in the tofacitinib 5 mg twice daily arm and 19% in the tofacitinib 10 mg twice daily arm at month 24.
- Mean HDL cholesterol increased by 17% in the tofacitinib 5 mg twice daily arm and 18% in the tofacitinib 10 mg twice daily arm at month 12, and increased by 19% in the tofacitinib 5 mg twice daily arm and 20% in the tofacitinib 10 mg twice daily arm at month 24.
Upon withdrawal of tofacitinib treatment, lipid levels returned to baseline.
Mean LDL cholesterol/HDL cholesterol ratios and Apolipoprotein B (ApoB)/ApoA1 ratios were essentially unchanged in tofacitinib-treated patients.
In an RA controlled clinical trial, elevations in LDL cholesterol and ApoB decreased to pretreatment levels in response to statin therapy.
In the RA long-term safety populations, elevations in the lipid parameters remained consistent with what was seen in the controlled clinical studies.
In the clinical studies in UC, changes in lipids observed with tofacitinib treatment were similar to the changes observed in clinical studies in RA.
Paediatric population
Polyarticular juvenile idiopathic arthritis and juvenile PsA
The adverse reactions in JIA patients in the clinical development program were consistent in type and frequency with those seen in adult RA patients, with the exception of some infections (influenza, pharyngitis, sinusitis, viral infection) and gastrointestinal or general disorders (abdominal pain, nausea, vomiting, pyrexia, headache, cough), which were more common in JIA paediatric population. MTX was the most frequent concomitant csDMARD used (on Day 1, 156 of 157 patients on csDMARDs took MTX). There are insufficient data regarding the safety profile of tofacitinib used concomitantly with any other csDMARDs.
Infections
In the double-blind portion of the pivotal Phase 3 trial (Study JIA-I), infection was the most commonly reported adverse reaction (44.3%). The infections were generally mild to moderate in severity.
In the integrated safety population, 7 patients had serious infections during treatment with tofacitinib within the reporting period (up to 28 days after the last dose of study medication), representing an incidence rate of 1.92 patients with events per 100 patient-years: pneumonia, epidural empyema (with sinusitis and subperiosteal abscess), pilonidal cyst, appendicitis, escherichia pyelonephritis, abscess limb, and UTI.
In the integrated safety population, 3 patients had non-serious events of herpes zoster within the reporting window representing an incidence rate of 0.82 patients with events per 100 patient-years. One (1) additional patient had an event of serious HZ outside the reporting window.
Hepatic events
Patients in the JIA pivotal study were required to have AST and ALT levels less than 1.5 times the upper limit of normal to be eligible for enrolment. In the integrated safety population, there were 2 patients with ALT elevations ≥3 times the ULN at 2 consecutive visits. Neither event met Hy's Law criteria. Both patients were on background MTX therapy and each event resolved after discontinuation of MTX and permanent discontinuation of tofacitinib.
Laboratory tests
Changes in laboratory tests in JIA patients in the clinical development program were consistent with those seen in adult RA patients. Patients in the JIA pivotal study were required to have a platelet count ≥100,000 cells/mm³ to be eligible for enrolment, therefore, there is no information available for JIA patients with a platelet count <100,000 cells/mm³ before starting treatment with tofacitinib.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.