Mecobalamin Other names: Methylcobalamin Vitamin MeB12
Chemical formula: C₆₃H₉₁CoN₁₃O₁₄P Molecular mass: 1,344.382 g/mol PubChem compound: 71306319
Pharmacodynamic properties
1) Mecobalamin is a kind of endogenous coenzyme B12.
Mecobalamin plays an important role in transmethylation as a coenzyme of methionine synthetase in the synthesis of methionine from homocysteine.
2) Mecobalamin is well transported to nerve cell organelles, and promotes nucleic acid and protein synthesis.
Mecobalamin is better transported to nerve cell organelles than cyanocobalamin in rats. It has been shown in experiments with cells from the brain origin and spinal nerve cells in rats to be involved in the synthesis of thymidine from deoxyuridine, promotion of deposited folic acid utilization and metabolism of nucleic acid. Also, mecobalamin promotes nucleic acid and protein synthesis in rats more than cobamamide does.
3) Mecobalamin promotes axonal transport and axonal regeneration.
Mecobalamin normalizes axonal skeletal protein transport in sciatic nerve cells from rat models with streptozotocin-induced diabetes mellitus. It exhibits neuropathologically and electrophysiologically inhibitory effects on nerve degeneration in neuropathies induced by drugs, such as adriamycin, acrylamide, and vincristine (in rats and rabbits), models of axonal degeneration in mice and neuropathies in rats with spontaneous diabetes mellitus.
4) Mecobalamin promotes myelination (phospholipid synthesis).
Mecobalamin promotes the synthesis of lecithin, the main constituent of medullary sheath lipids, and increases myelination of neurons in rat tissue culture more than cobamamide does.
5) Mecobalamin restores delayed synaptic transmission and diminished neurotransmitters to normal.
Mecobalamin restores end-plate potential induction early by increasing nerve fiber excitability in the crushed sciatic nerve in rats. In addition, mecobalamin normalizes diminished brain tissue levels of acetylcholine in rats fed a choline-deficient diet.
6) Mecobalamin promotes the maturation and division of erythroblasts, thereby improving anemia. Mecobalamin promotes nucleic acid synthesis in the bone marrow and promotes the maturation and division of erythroblasts, thus increasing erythrocyte production. Mecobalamin brings about a rapid recovery of diminished WBC, hemoglobin, and hematocrit in vitamin B12-deficient rats.
Pharmacokinetic properties
Single-dose administration
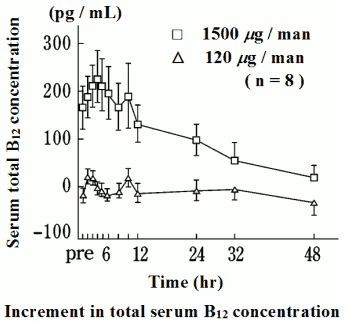
When mecobalamin was administered orally to healthy adult male volunteers at single doses of 120 μg and 1,500 µgnote)* during fasting, the peak serum total vitamin B12 (abbreviated to B12) concentration was reached after 3 hrs for both doses, and this was dose-dependent. The half-life, increment in the serum total B12 concentration and ΔAUC102 by 12 hrs after administration are shown in the following figure and table. 40 to 90% of the cumulative amount of total B12 excreted in the urine by 24 hrs after administration was excreted within the first 8 hrs.
Note: The single doses of 1,500 μg marked with the symbol* are unapproved.
| Dose | tmax (hr) | Cmax (pg/mL) | ΔCmax (pg/mL) | ΔCmax (%) | ΔAUC (pg·hr/mL) | t1/2*2 (hr) |
|---|---|---|---|---|---|---|
| 120 μg | 2.8±0.2 | 743±47 | 37±15 | 5.1±2.1 | 168±58 | N.A. |
| 1500 μg | 3.6±0.5 | 972±55 | 255±51 | 36.0±7.9 | 2033±510 | 12.5 |
Mean±S.E., n=8
*1 Calculated by the trapezoidal method from the increment in observed 12 hr values, as compared to pre-drug values.
*2 Calculated from the average of 24-48 hr values.
When a single i.m. or i.v. Of 500μg of mecobalamin was administered to healthy adult volunteers, the time required for the serum total vitamin B12 level to reach (tmax) was 0.9 ± .1 hour after i.m. administration and immediately to 3 min. after i.v. administration, and the increment in the peak serum total vitamin B12 level (ΔCmax) was 22.4 ± 1.1 ng/mL after i.m. dministration and 85.0 ± 8.9ng/ml after i.v. administration.
The area under the blood concentration-time curve (ΔAUC) at 144 hours after administration was 204.1 ± 12.9 hr•ng/ml after i.m. administration and 358.6 ± 34.4 hr•ng/ml after i.v. administration. On the other hand, the rate of binding saturation showed a similar increase in both groups of subjects for 144 hours after administration.
Repeated-dose administration
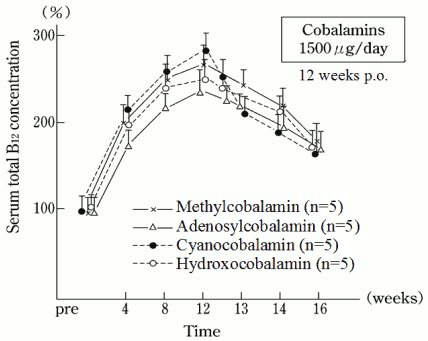
Mecobalamin was administered orally to healthy adult male volunteers at a dose of 1,500 μg daily for 12 consecutive weeks and changes in the serum total B12 concentration were determined until 4 weeks after the last administration. The serum concentration increased for the first 4 weeks after administration, rising to about twice as high as the initial value. Thereafter, there was a gradual increase, which peaked at about 2.8 times the initial value at the 12th week of dosing. The serum concentration declined after the last administration (12 weeks), but was still about 1.8 times the initial value 4 weeks after the last administration.
500μg/day of CH3-B12 was administered intravenously to healthy adults for 10 consecutive days. Serum total vitamin 1312 levels measured before each administration (ΔCmin) increased from day to day. The serum level of total vitamin B12 24 hours after the administration on the day 2nd was 5.3 1.8ng/ml, about 1.4 times the 24 hour-value (3.9 ± 1.2 ng/ml) of the day 1st.
On the 3rd, it increased to 6.8 ± 1.5 ng/ml, about 1.7 times the 24-hour value of the day 1st, and this level was maintained until the last dosing.
Preclinical safety data
Clinical efficacy
In a double-blind comparison clinical trial with a low-dose group (100μg), the efficacy of mecobalamin in peripheral neuropathies in the chronic fixed stage was demonstrated.
In a placebo-controlled double-blind study trial, equivalence of mecobalamin between the intravenous and the intramuscular routes of administration was shown. When mecobalamin was administered to patients with vitamin B12 deficiency anemia, symptoms and hemogram improved.
Animal studies
Distribution
In rat given a 25 μg oral dose of 57Co-labeled mecobalamin, radioactivity was found to be higher in the kidneys, adrenal glands, pancreas, liver, and stomach (listed in order of magnitude), whereas the muscles, testes, brain, and nerves did not show significant radioactivity 72 hours after administration.
At 24 hours after i.v. administration of 57Co-CH3-B12 to rats in a dose of 10μg/kg, the radioactivity was detected in order of high concentration in the kidneys, adrenal, intestine, pancreas and pituitary, and the concentration of radioactivity was relatively lower in the eyes, spinal cord, brain and muscle.
Acute toxicity LD50 (mg/kg)
| Animal | i.v. | p.o. |
|---|---|---|
| Mice | >666 | >1,000 |
| Rats | >333 | >500 |
| Rabbits | >60 | - |
| Beagles | >200 | - |
Subacute toxicity
The oral administration of mecobalamin, at doses of 0.2, 2.0 and 20.0 mg/kg/day to rats of both sexes for 1 consecutive month, resulted in no remarkable changes in the general condition of the animals, the body weights, blood analytical results, organ weight, or histopathology.
Mecobalamin was administered intravenously to dogs in doses of 0.5, 5.0 and 50.0
mg/kg/day for 90 consecutive days. Changes in general conditions, body weight, blood and organ weights were unremarkable in all the dose groups. Histopathologically, increases in eosinophils and lysosomes were found in the observation with an optical microscope, and an electron microscope in the 50.0 mg/kg or higher dose respectively, in the epithelial cell of proximal renal tubule in the animals of the 50.0 mg/kg/day dose group. No change, however, was noted in the epididymides
Chronic toxicity
In rats of both sexes, given an oral dose of 0.2, 2.0 and 20.0 mg/kg/day of mecobalamin for 6 consecutive months, no remarkable changes were noted in the general condition of the animals, the body weights, blood and urine analytical results, organ weights, or histopathology.
Mecobalamin was administered intravenously to dogs at doses of 0.5, 5.0 and 50.0
mg/kg/day for 12 consecutive months. Changes in general condition, body weight, blood and organ weights were unremarkable in all the dose groups of beagles. Histopathologically, increases in eosinophils and lysosomes were found in the observation with an optical microscope and an electron microscope, respectively, in the epithelial cells of proximal renal tubule in the 5.0 mg/kg/day or higher dose groups. In the 50.0 mg/kg/day dose groups lysosomes in the mesangial cells of the renal glomerulus and Küpferr cells in the liver were increased.
Reproductive and developmental toxicity
In mature nulliparous mice and rats, administered an oral dose of 0.2, 2.0 and 20.0 mg/kg/day of mecobalamin during organogenesis, no abnormalities or signs of teratogenicity were noted in fetuses or neonates.
Mecobalamin was administered intravenously in doses of 0.5, 5.0 and 50 mg/kg/day to rats prior to and, in the early stages of pregnancy, during the period of organogenesis, and during the perinatal and lactating periods. No abnormal findings nor signs of teratogenicity were observed in the fetuses and newborns from these dams.
Methylation of inorganic mercury
In vitro, a chemical reaction occurs such that mecobalamin forms methyl mercury with mercuric chloride. The reaction, however, does not take place in the presence of proteins as in the human blood.
Experiments with rats fed a diet containing inorganic mercury have shown clearly that oral administration of mecobalamin does not increase the formation of methyl mercury in the body. In male rats fed a diet containing inorganic mercury, mecobalamin administered in oral doses of 1.5 and 15 mg/kg/day for 6 consecutive months did not affect the general condition of the animals, and no toxic signs were observed with urinalysis nor from haematological or histopathological examinations.
Related medicines
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.