NEURONTIN Capsule / Film-coated tablet / Solution Ref.[10600] Active ingredients: Gabapentin
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
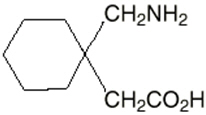
The active ingredient in NEURONTIN capsules, tablets, and oral solution is gabapentin, which has the chemical name 1-(aminomethyl)cyclohexaneacetic acid.
The molecular formula of gabapentin is C9H17NO2 and the molecular weight is 171.24. The structural formula of gabapentin is:
Gabapentin is a white to off-white crystalline solid with a pKa1 of 3.7 and a pKa2 of 10.7. It is freely soluble in water and both basic and acidic aqueous solutions. The log of the partition coefficient (n-octanol/0.05M phosphate buffer) at pH 7.4 is -1.25.
Each NEURONTIN capsule contains 100 mg, 300 mg, or 400 mg of gabapentin and the following inactive ingredients: lactose, cornstarch, talc, gelatin, titanium dioxide, FD&C Blue No. 2, yellow iron oxide (300 mg and 400 mg only), and red iron oxide (400 mg only).
Each NEURONTIN tablet contains 600 mg or 800 mg of gabapentin and the following inactive ingredients: poloxamer 407, copovidone, cornstarch, magnesium stearate, hydroxypropyl cellulose, talc, and candelilla wax
NEURONTIN oral solution contains 250 mg of gabapentin per 5 mL (50 mg per mL) and the following inactive ingredients: glycerin, xylitol, purified water, and artificial cool strawberry anise flavor.
| Dosage Forms and Strengths |
|---|
Capsules
Tablets
Oral solution
|
| How Supplied |
|---|
|
NEURONTIN (gabapentin) capsules, tablets, and oral solution are supplied as follows: 100 mg capsules: White hard gelatin capsules printed with “PD” on the body and “Neurontin/100 mg” on the cap; available in: Bottles of 100: NDC 0071-0803-24 300 mg capsules: Yellow hard gelatin capsules printed with “PD” on the body and “Neurontin/300 mg” on the cap; available in: Bottles of 100: NDC 0071-0805-24 400 mg capsules: Orange hard gelatin capsules printed with “PD” on the body and “Neurontin/400 mg” on the cap; available in: Bottles of 100: NDC 0071-0806-24 600 mg tablets: White elliptical film-coated scored tablets debossed with “NT” and “16” on one side; available in: Bottles of 100: NDC 0071-0513-24 800 mg tablets: White elliptical film-coated scored tablets debossed with “NT” and “26” on one side; available in: Bottles of 100: NDC 0071-0401-24 250 mg per 5 mL oral solution: Clear colorless to slightly yellow solution; each 5 mL of oral solution contains 250 mg of gabapentin; available in: Glass bottles containing 470 mL: NDC 0071-2012-23 |
Drugs
| Drug | Countries | |
|---|---|---|
| NEURONTIN | Austria, Australia, Brazil, Canada, Cyprus, Germany, Ecuador, Estonia, Spain, Finland, France, Hong Kong, Croatia, Ireland, Israel, Italy, Lithuania, Mexico, Netherlands, New Zealand, Poland, Singapore, Tunisia, Turkey, United Kingdom, United States, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.