Prezista 75 mg, 150 mg, 400 mg, 600 mg film-coated tablets and Oral suspension 100mg/ml Ref.[2703] Active ingredients: Darunavir
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2012 Publisher: Janssen-Cilag International NV Turnhoutseweg 30 B-2340 Beerse Belgium
Pharmacodynamic properties
Pharmacotherapeutic group: Antivirals for systemic use, protease inhibitors
ATC code: J05AE10
Mechanism of action
Darunavir is an inhibitor of the dimerisation and of the catalytic activity of the HIV-1 protease (KD of 4.5 × 10-12M). It selectively inhibits the cleavage of HIV encoded Gag-Pol polyproteins in virus infected cells, thereby preventing the formation of mature infectious virus particles.
Antiviral activity in vitro
Darunavir exhibits activity against laboratory strains and clinical isolates of HIV-1 and laboratory strains of HIV-2 in acutely infected T-cell lines, human peripheral blood mononuclear cells and human monocytes/macrophages with median EC50 values ranging from 1.2 to 8.5 nM (0.7 to 5.0 ng/ml). Darunavir demonstrates antiviral activity in vitro against a broad panel of HIV-1 group M (A, B, C, D, E, F, G) and group O primary isolates with EC50 values ranging from < 0.1 to 4.3 nM.
These EC50 values are well below the 50% cellular toxicity concentration range of 87 µM to > 100 µM.
Resistance
In vitro selection of darunavir-resistant virus from wild type HIV-1 was lengthy (> 3 years). The selected viruses were unable to grow in the presence of darunavir concentrations above 400 nM. Viruses selected in these conditions and showing decreased susceptibility to darunavir (range: 23-50-fold) harboured 2 to 4 amino acid substitutions in the protease gene. Identification of determinants of decreased susceptibility to darunavir in those viruses is under investigation.
The clinical trial data from ART-experienced patients (TITAN trial and the pooled analysis of the POWER 1, 2 and 3 and DUET 1 and 2 trials) showed that virologic response to PREZISTA co-administered with low dose ritonavir was decreased when 3 or more darunavir RAMs (V11I, V32I, L33F, I47V, I50V, I54L or M, T74P, L76V, I84V and L89V) were present at baseline or when these mutations developed during treatment.
Increasing baseline darunavir fold change in EC50 (FC) was associated with decreasing virologic response. A lower and upper clinical cut-off of 10 and 40 were identified. Isolates with baseline FC ≤ 10 are susceptible; isolates with FC > 10 to 40 have decreased susceptibility; isolates with FC > 40 are resistant (see Clinical results).
Viruses isolated from patients on PREZISTA/rtv 600/100 mg twice daily experiencing virologic failure by rebound that were susceptible to tipranavir at baseline remained susceptible to tipranavir after treatment in the vast majority of cases.
The lowest rates of developing resistant HIV virus are observed in ART-naïve patients who are treated for the first time with darunavir in combination with other ART.
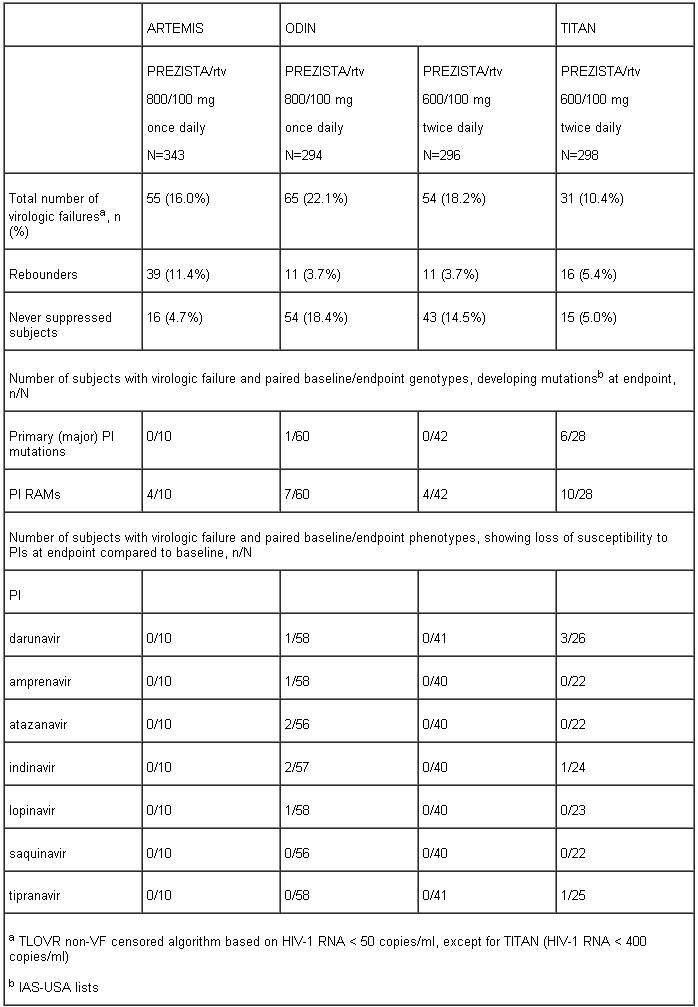
The table below shows the development of mutations and loss of susceptibility to PIs in virologic failures at endpoint in the ARTEMIS, ODIN and TITAN trials.
Cross-resistance
Darunavir FC was less than 10 for 90% of 3,309 clinical isolates resistant to amprenavir, atazanavir, indinavir, lopinavir, nelfinavir, ritonavir, saquinavir and/or tipranavir showing that viruses resistant to most PIs remain susceptible to darunavir.
In the virologic failures of the ARTEMIS trial no cross-resistance with other PIs was observed.
Clinical results
Adult patients
Efficacy of PREZISTA 800 mg once daily co-administered with 100 mg ritonavir once daily in ART-naïve patients
The evidence of efficacy of PREZISTA/ritonavir 800/100 mg once daily is based on the analyses of 192 week data from the randomised, controlled, open-label Phase III trial ARTEMIS in antiretroviral treatment-naïve HIV-1 infected patients comparing PREZISTA/ritonavir 800/100 mg once daily with lopinavir/ritonavir 800/200 mg per day (given as a twice-daily or as a once-daily regimen). Both arms used a fixed background regimen consisting of tenofovir disoproxil fumarate 300 mg once daily and emtricitabine 200 mg once daily.
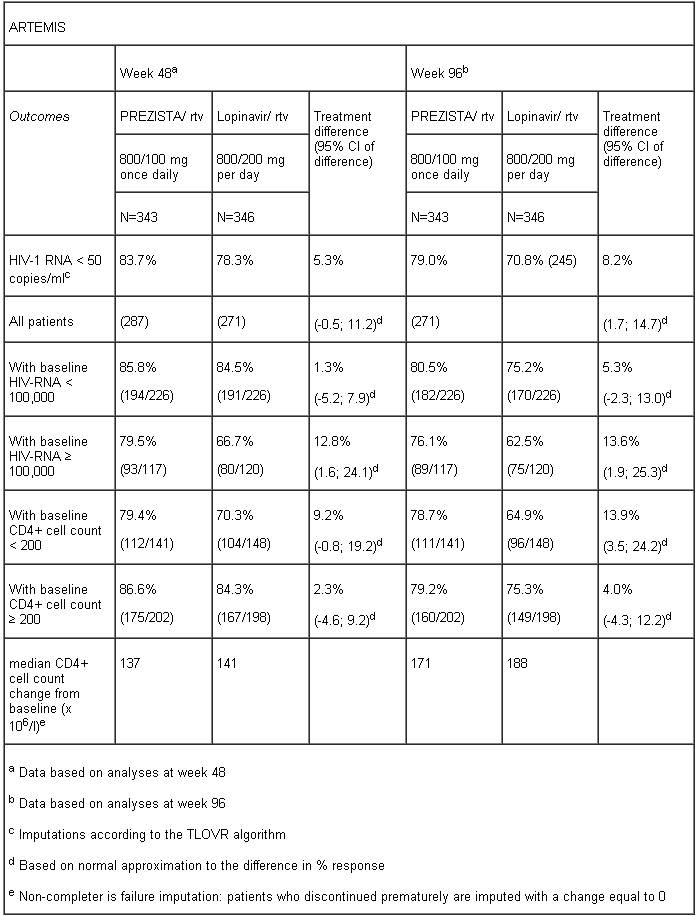
The table below shows the efficacy data of the 48 week and 96 week analyses from the ARTEMIS trial:
Non-inferiority in virologic response to the PREZISTA/ritonavir treatment, defined as the percentage of patients with plasma HIV-1 RNA level < 50 copies/ml, was demonstrated (at the pre-defined 12% non-inferiority margin) for both Intent-To-Treat (ITT) and On Protocol (OP) populations in the 48 week analysis. These results were confirmed in the analyses of data at 96 weeks of treatment in the ARTEMIS trial. These results were sustained up to 192 weeks of treatment in the ARTEMIS trial.
Efficacy of PREZISTA 800 mg once daily co-administered with 100 mg ritonavir once daily in ART-experienced patients
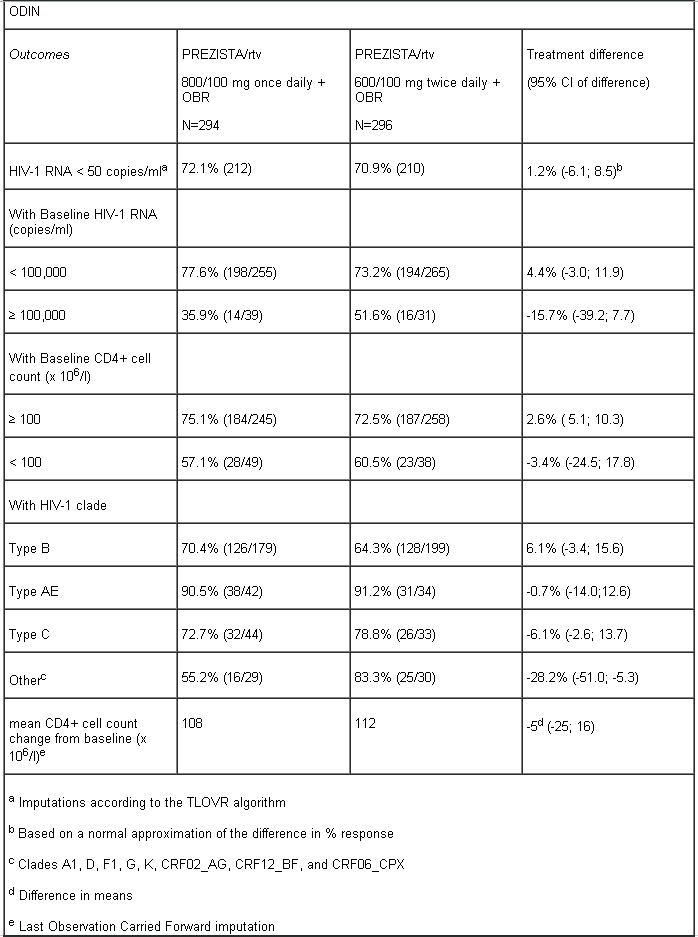
ODIN is a Phase III, randomised, open-label trial comparing PREZISTA/rtv 800/100 mg once daily versus PREZISTA/rtv 600/100 mg twice daily in ART-experienced HIV-1 infected patients with screening genotype resistance testing showing no darunavir RAMs (i.e. V11I, V32I, L33F, I47V, I50V, I54M, I54L, T74P, L76V, I84V, L89V) and a screening HIV-1 RNA > 1,000 copies/ml. Efficacy analysis is based on 48 weeks of treatment (see table below). Both arms used an optimised background regimen (OBR) of ≥ 2 NRTIs.
At 48 weeks, virologic response, defined as the percentage of patients with plasma HIV-1 RNA level < 50 copies/ml, with PREZISTA/ritonavir 800/100 mg once daily treatment was demonstrated to be non-inferior (at the pre-defined 12% non-inferiority margin) compared to PREZISTA/ritonavir 600/100 mg twice daily for both ITT and OP populations.
PREZISTA/rtv 800/100 mg once daily in ART-experienced patients should not be used in patients with one or more darunavir resistance associated mutations (DRV-RAMs) or HIV-1 RNA ≥ 100,000 copies/ml or CD4+ cell count < 100 cells x 106/l (see section 4.2 and 4.4). Limited data is available in patients with HIV-1 clades other than B.
Efficacy of PREZISTA 600 mg twice daily co-administered with 100 mg ritonavir twice daily in ART-experienced patients
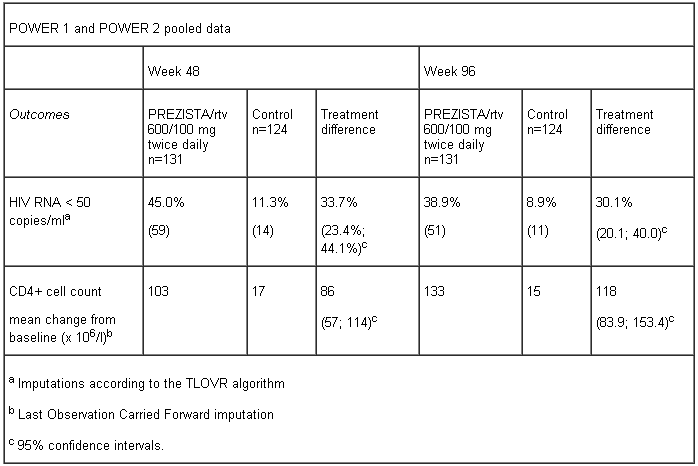
The evidence of efficacy of PREZISTA co-administered with ritonavir (600/100 mg twice daily) in ART-experienced patients is based on the 96 weeks analysis of the Phase III trial TITAN in ART-experienced lopinavir naïve patients, on the 48 week analysis of the Phase III trial ODIN in ART-experienced patients with no DRV-RAMs, and on the analyses of 96 weeks data from the Phase IIb trials POWER 1 and 2 in ART-experienced patients with high level of PI resistance.
TITAN is a randomised, controlled, open-label Phase III trial comparing PREZISTA co-administered with ritonavir (600/100 mg twice daily) versus lopinavir/ritonavir (400/100 mg twice daily) in ART-experienced, lopinavir naïve HIV-1 infected adult patients. Both arms used an Optimised Background Regimen (OBR) consisting of at least 2 antiretrovirals (NRTIs with or without NNRTIs).
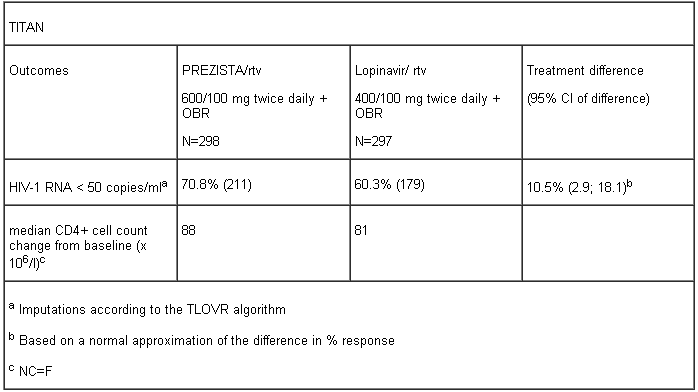
The table below shows the efficacy data of the 48 week analysis from the TITAN trial.
At 48 weeks non-inferiority in virologic response to the PREZISTA/ritonavir treatment, defined as the percentage of patients with plasma HIV-1 RNA level < 400 and < 50 copies/ml, was demonstrated (at the pre-defined 12% non-inferiority margin) for both ITT and OP populations. These results were confirmed in the analysis of data at 96 weeks of treatment in the TITAN trial, with 60.4% of patients in the PREZISTA/rtv arm having HIV-1 RNA < 50 copies/ml at week 96 compared to 55.2% in the lopinavir/rtv arm [difference: 5.2%, 95% CI (-2.8;13.1)].
POWER 1 and POWER 2 are randomised, controlled trials comparing PREZISTA co-administered with ritonavir (600/100 mg twice daily) with a control group receiving an investigator-selected PI(s) regimen in HIV-1 infected patients who had previously failed more than 1 PI containing regimen. An OBR consisting of at least 2 NRTIs with or without enfuvirtide (ENF) was used in both trials.
The table below shows the efficacy data of the 48-week and 96-week analyses from the pooled POWER 1 and POWER 2 trials.
Analyses of data through 96 weeks of treatment in the POWER trials demonstrated sustained antiretroviral efficacy and immunologic benefit.
Out of the 59 patients who responded with complete viral suppression (< 50 copies/ml) at week 48, 47 patients (80% of the responders at week 48) remained responders at week 96.
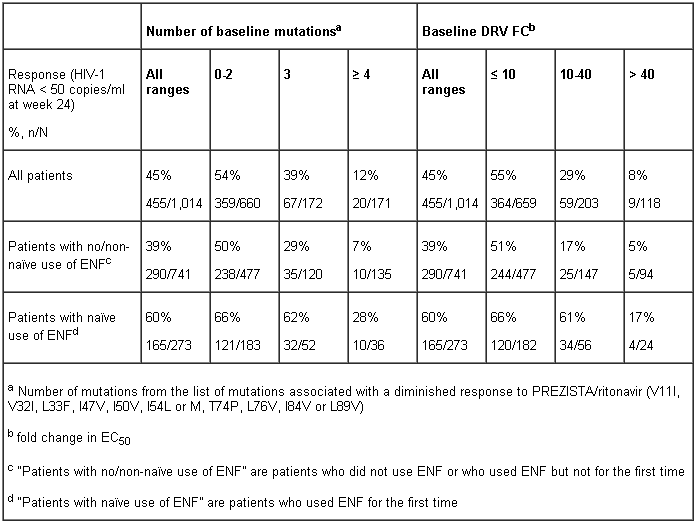
Baseline genotype or phenotype and virologic outcome
Baseline genotype and darunavir FC (shift in susceptibility relative to reference) were shown to be a predictive factor of virologic outcome.
Proportion (%) of patients with response (HIV-1 RNA < 50 copies/ml at week 24) to PREZISTA co-administered with ritonavir (600/100 mg twice daily) by baseline genotypea, and baseline darunavir FC and by use of enfuvirtide (ENF): As treated analysis of the POWER and DUET trials
Description of clinical studies in paediatric patients
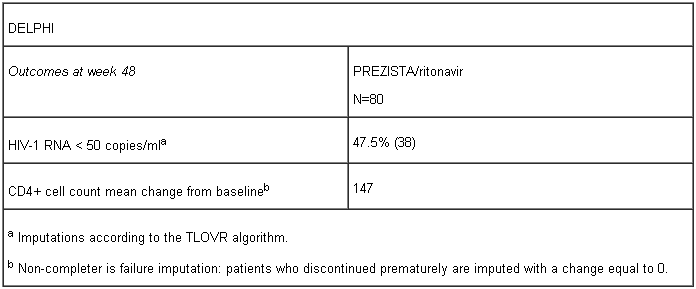
The evidence of efficacy of PREZISTA/rtv in treatment-experienced paediatric patients is based on the Phase II trial DELPHI in paediatric patients aged 6 to < 18 years and weighing at least 20 kg as well as ARIEL in patients aged 3 to < 6 years and weighing ≥ 15 kg to < 20 kg.
Paediatric patients from the age of 6 to < 18 years
DELPHI is an open-label, Phase II trial evaluating the pharmacokinetics, safety, tolerability, and efficacy of PREZISTA with low dose ritonavir in 80 ART-experienced HIV-1 infected paediatric patients aged 6 to 17 years and weighing at least 20 kg. These patients received PREZISTA/ritonavir in combination with other antiretroviral agents (see section 4.2 for dosage recommendations per body weight). Virologic response was defined as a decrease in plasma HIV-1 RNA viral load of at least 1.0 log10 versus baseline.
In the study, patients who were at risk of discontinuing therapy due to intolerance of ritonavir oral solution (e.g. taste aversion) were allowed to switch to the capsule formulation. Of the 44 patients taking ritonavir oral solution, 27 switched to the 100 mg capsule formulation and exceeded the weight-based ritonavir dose without changes in observed safety.
According to the TLOVR non-virologic failure censored algorithm 24 (30.0%) patients experienced virological failure, of which 17 (21.3%) patients were rebounders and 7 (8.8%) patients were non-responders.
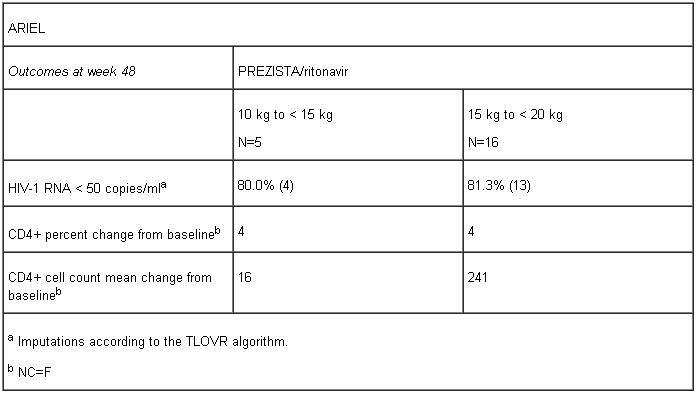
Paediatric patients from the age of 3 to < 6 years
The pharmacokinetics, safety, tolerability and efficacy of PREZISTA/rtv in combination with other antiretroviral agents in 21 ART-experienced HIV-1 infected paediatric patients aged 3 to < 6 years and weighing 10 kg to < 20 kg was evaluated in an open-label, Phase II trial, ARIEL. Patients received a weight-based twice daily treatment regimen, patients weighing 10 kg to < 15 kg received darunavir/rtv 25/3 mg/kg b.i.d, and patients weighing 15 kg to < 20 kg received darunavir/rtv 375/50 mg b.i.d. At week 48, the virologic response, defined as the percentage of patients with confirmed plasma viral load < 50 HIV-1 RNA copies/ml, was evaluated in 16 paediatric patients 15 kg to < 20 kg and 5 paediatric patients 10 kg to < 15 kg receiving PREZISTA/rtv in combination with other antiretroviral agents (see section 4.2 for dosage recommendations per body weight).
Limited efficacy data are available in paediatric patients below 15 kg and no recommendation on a posology can be made.
The European Medicines Agency has deferred the obligation to submit the results of studies with PREZISTA in one or more subsets of the paediatric population in Human Immunodeficiency Virus infection, as per Paediatric Investigation Plan (PIP) decision in the granted indication (see section 4.2 for information on paediatric use).
Pharmacokinetic properties
The pharmacokinetic properties of darunavir, co-administered with ritonavir, have been evaluated in healthy adult volunteers and in HIV-1 infected patients. Exposure to darunavir was higher in HIV-1 infected patients than in healthy subjects. The increased exposure to darunavir in HIV-1 infected patients compared to healthy subjects may be explained by the higher concentrations of α1-acid glycoprotein (AAG) in HIV-1 infected patients, resulting in higher darunavir binding to plasma AAG and, therefore, higher plasma concentrations.
Darunavir is primarily metabolised by CYP3A. Ritonavir inhibits CYP3A, thereby increasing the plasma concentrations of darunavir considerably.
Absorption
Darunavir was rapidly absorbed following oral administration. Maximum plasma concentration of darunavir in the presence of low dose ritonavir is generally achieved within 2.5-4.0 hours.
The absolute oral bioavailability of a single 600 mg dose of darunavir alone was approximately 37% and increased to approximately 82% in the presence of 100 mg twice daily ritonavir. The overall pharmacokinetic enhancement effect by ritonavir was an approximate 14-fold increase in the systemic exposure of darunavir when a single dose of 600 mg darunavir was given orally in combination with ritonavir at 100 mg twice daily (see section 4.4).
When administered without food, the relative bioavailability of darunavir in the presence of low dose ritonavir is 30% lower as compared to intake with food. Therefore, PREZISTA tablets should be taken with ritonavir and with food. The type of food does not affect exposure to darunavir.
Distribution
Darunavir is approximately 95% bound to plasma protein. Darunavir binds primarily to plasma α1-acid glycoprotein.
Following intravenous administration, the volume of distribution of darunavir alone was 88.1 ± 59.0 l (Mean ± SD) and increased to 131 ± 49.9 l (Mean ± SD) in the presence of 100 mg twice-daily ritonavir.
Biotransformation
In vitro experiments with human liver microsomes (HLMs) indicate that darunavir primarily undergoes oxidative metabolism. Darunavir is extensively metabolised by the hepatic CYP system and almost exclusively by isozyme CYP3A4. A 14C-darunavir trial in healthy volunteers showed that a majority of the radioactivity in plasma after a single 400/100 mg darunavir with ritonavir dose was due to the parent active substance. At least 3 oxidative metabolites of darunavir have been identified in humans; all showed activity that was at least 10-fold less than the activity of darunavir against wild type HIV.
Elimination
After a 400/100 mg 14C-darunavir with ritonavir dose, approximately 79.5% and 13.9% of the administered dose of 14C-darunavir could be retrieved in faeces and urine, respectively. Unchanged darunavir accounted for approximately 41.2% and 7.7% of the administered dose in faeces and urine, respectively. The terminal elimination half-life of darunavir was approximately 15 hours when combined with ritonavir.
The intravenous clearance of darunavir alone (150 mg) and in the presence of low dose ritonavir was 32.8 l/h and 5.9 l/h, respectively.
Special populations
Paediatric population
The pharmacokinetics of darunavir in combination with ritonavir in 74 treatment-experienced paediatric patients, aged 6 to 17 years and weighing at least 20 kg, showed that the administered weight-based doses of PREZISTA/ritonavir resulted in darunavir exposure comparable to that in adults receiving PREZISTA/ritonavir 600/100 mg twice daily (see section 4.2).
The pharmacokinetics of darunavir in combination with ritonavir in 14 treatment-experienced paediatric patients, aged 3 to < 6 years and weighing at least 15 kg to < 20 kg, showed that weight-based dosages resulted in darunavir exposure that was comparable to that achieved in adults receiving PREZISTA/rtv 600/100 mg twice daily (see section 4.2).
Elderly
Population pharmacokinetic analysis in HIV infected patients showed that darunavir pharmacokinetics are not considerably different in the age range (18 to 75 years) evaluated in HIV infected patients (n=12, age ≥ 65) (see section 4.4). However, only limited data were available in patients above the age of 65 year.
Gender
Population pharmacokinetic analysis showed a slightly higher darunavir exposure (16.8%) in HIV infected females compared to males. This difference is not clinically relevant.
Renal impairment
Results from a mass balance study with 14C-darunavir with ritonavir showed that approximately 7.7% of the administered dose of darunavir is excreted in the urine unchanged.
Although darunavir has not been studied in patients with renal impairment, population pharmacokinetic analysis showed that the pharmacokinetics of darunavir were not significantly affected in HIV infected patients with moderate renal impairment (CrCl between 30-60 ml/min, n=20) (see sections 4.2 and 4.4).
Hepatic impairment
Darunavir is primarily metabolised and eliminated by the liver. In a multiple dose study with PREZISTA co-administered with ritonavir (600/100 mg) twice daily, it was demonstrated that the total plasma concentrations of darunavir in subjects with mild (Child-Pugh Class A, n=8) and moderate (Child-Pugh Class B, n=8) hepatic impairment were comparable with those in healthy subjects. However, unbound darunavir concentrations were approximately 55% (Child-Pugh Class A) and 100% (Child-Pugh Class B) higher, respectively. The clinical relevance of this increase is unknown therefore, PREZISTA should be used with caution. The effect of severe hepatic impairment on the pharmacokinetics of darunavir has not been studied (see sections 4.2, 4.3 and 4.4).
Preclinical safety data
Animal toxicology studies have been conducted at exposures up to clinical exposure levels with darunavir alone, in mice, rats and dogs and in combination with ritonavir in rats and dogs.
In repeated-dose toxicology studies in mice, rats and dogs, there were only limited effects of treatment with darunavir. In rodents the target organs identified were the haematopoietic system, the blood coagulation system, liver and thyroid. A variable but limited decrease in red blood cell-related parameters was observed, together with increases in activated partial thromboplastin time.
Changes were observed in liver (hepatocyte hypertrophy, vacuolation, increased liver enzymes) and thyroid (follicular hypertrophy). In the rat, the combination of darunavir with ritonavir lead to a small increase in effect on RBC parameters, liver and thyroid and increased incidence of islet fibrosis in the pancreas (in male rats only) compared to treatment with darunavir alone. In the dog, no major toxicity findings or target organs were identified up to exposures equivalent to clinical exposure at the recommended dose.
In a study conducted in rats, the number of corpora lutea and implantations were decreased in the presence of maternal toxicity. Otherwise, there were no effects on mating or fertility with darunavir treatment up to 1,000 mg/kg/day and exposure levels below (AUC-0.5 fold) of that in human at the clinically recommended dose. Up to same dose levels, there was no teratogenicity with darunavir in rats and rabbits when treated alone nor in mice when treated in combination with ritonavir. The exposure levels were lower than those with the recommended clinical dose in humans. In a pre- and postnatal development assessment in rats, darunavir with and without ritonavir, caused a transient reduction in body weight gain of the offspring pre-weaning and there was a slight delay in the opening of eyes and ears. Darunavir in combination with ritonavir caused a reduction in the number of pups that exhibited the startle response on day 15 of lactation and a reduced pup survival during lactation. These effects may be secondary to pup exposure to the active substance via the milk and/or maternal toxicity. No post weaning functions were affected with darunavir alone or in combination with ritonavir. In juvenile rats receiving darunavir up to days 23-26, increased mortality was observed with convulsions in some animals. Exposure in plasma, liver and brain was considerably higher than in adult rats after comparable doses in mg/kg between days 5 and 11 of age. After day 23 of life, the exposure was comparable to that in adult rats. The increased exposure was likely at least partly due to immaturity of the drug-metabolising enzymes in juvenile animals. No treatment related mortalities were noted in juvenile rats dosed at 1,000 mg/kg darunavir (single dose) on day 26 of age or at 500 mg/kg (repeated dose) from day 23 to 50 of age, and the exposures and toxicity profile were comparable to those observed in adult rats.
Due to uncertainties regarding the rate of development of the human blood brain barrier and liver enzymes, PREZISTA with low dose ritonavir should not be used in paediatric patients below 3 years of age.
Darunavir was evaluated for carcinogenic potential by oral gavage administration to mice and rats up to 104 weeks. Daily doses of 150, 450 and 1,000 mg/kg were administered to mice and doses of 50, 150 and 500 mg/kg were administered to rats. Dose-related increases in the incidences of hepatocellular adenomas and carcinomas were observed in males and females of both species. Thyroid follicular cell adenomas were noted in male rats. Administration of darunavir did not cause a statistically significant increase in the incidence of any other benign or malignant neoplasm in mice or rats. The observed hepatocellular and thyroid tumours in rodents are considered to be of limited relevance to humans. Repeated administration of darunavir to rats caused hepatic microsomal enzyme induction and increased thyroid hormone elimination, which predispose rats, but not humans, to thyroid neoplasms. At the highest tested doses, the systemic exposures (based on AUC) to darunavir were between 0.4- and 0.7-fold (mice) and 0.7- and 1-fold (rats), relative to those observed in humans at the recommended therapeutic doses.
After 2 years administration of darunavir at exposures at or below the human exposure, kidney changes were observed in mice (nephrosis) and rats (chronic progressive nephropathy).
Darunavir was not mutagenic or genotoxic in a battery of in vitro and in vivo assays including bacterial reverse mutation (Ames), chromosomal aberration in human lymphocytes and in vivo micronucleus test in mice.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.