Prezista 75 mg, 150 mg, 400 mg, 600 mg film-coated tablets and Oral suspension 100mg/ml Ref.[2703] Active ingredients: Darunavir
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2012 Publisher: Janssen-Cilag International NV Turnhoutseweg 30 B-2340 Beerse Belgium
Contraindications
Hypersensitivity to the active substance or to any of the excipients listed in section 6.1.
Patients with severe (Child-Pugh Class C) hepatic impairment.
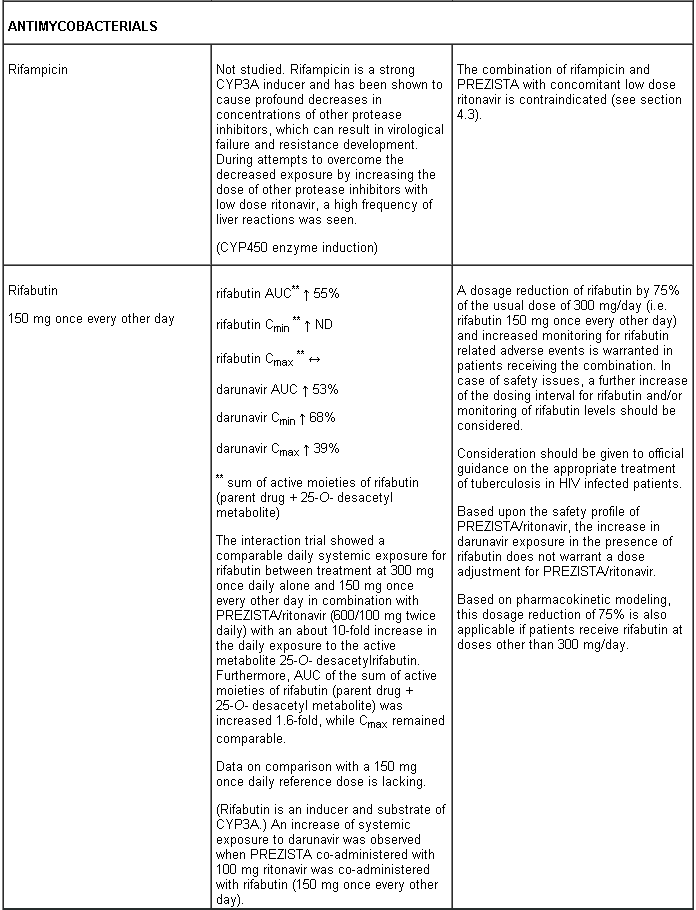
Combination of rifampicin with PREZISTA with concomitant low dose ritonavir is contraindicated (see section 4.5).
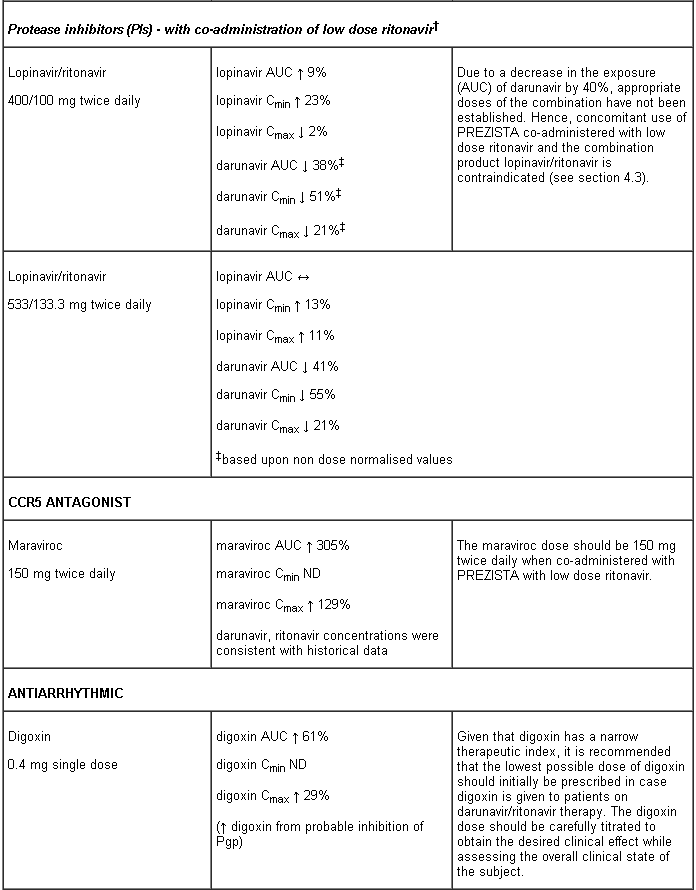
Co-administration with the combination product lopinavir/ritonavir (see section 4.5).
Co-administration with herbal preparations containing St John’s wort (Hypericum perforatum) (see section 4.5).
Co-administration of PREZISTA with low dose ritonavir, with active substances that are highly dependent on CYP3A for clearance and for which elevated plasma concentrations are associated with serious and/or life-threatening events. These active substances include e.g.
- antiarrhythmics (amiodarone, bepridil, quinidine, systemic lidocaine)
- alfuzosin
- antihistamines (astemizole, terfenadine)
- ergot derivatives (e.g. dihydroergotamine, ergonovine, ergotamine, methylergonovine)
- gastrointestinal motility agents (cisapride)
- neuroleptics (pimozide, sertindole)
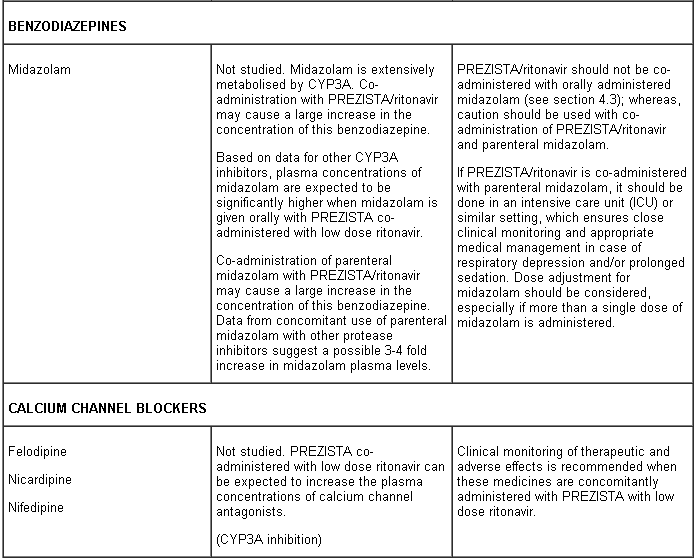
- sedatives/hypnotics [triazolam, midazolam administered orally (for caution on parenterally administered midazolam, see section 4.5)]
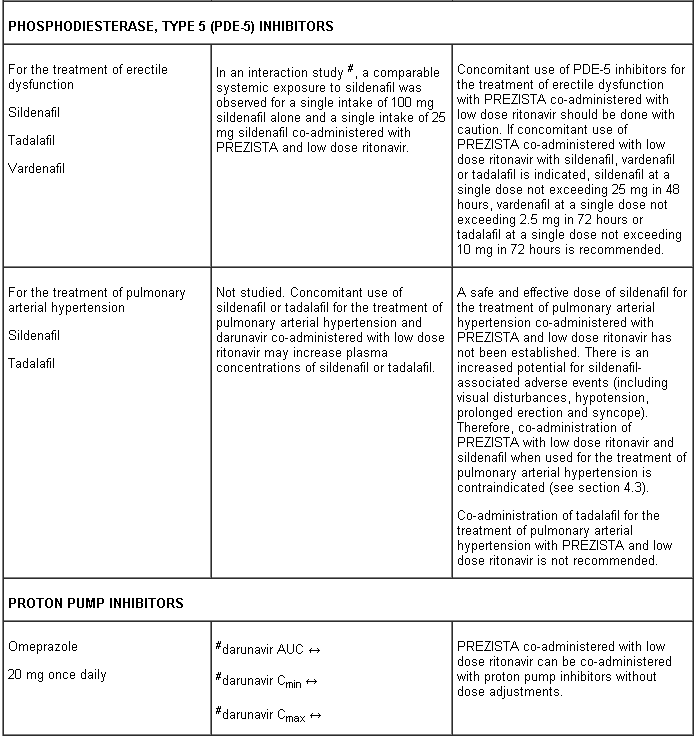
- sildenafil (when used for the treatment of pulmonary arterial hypertension)
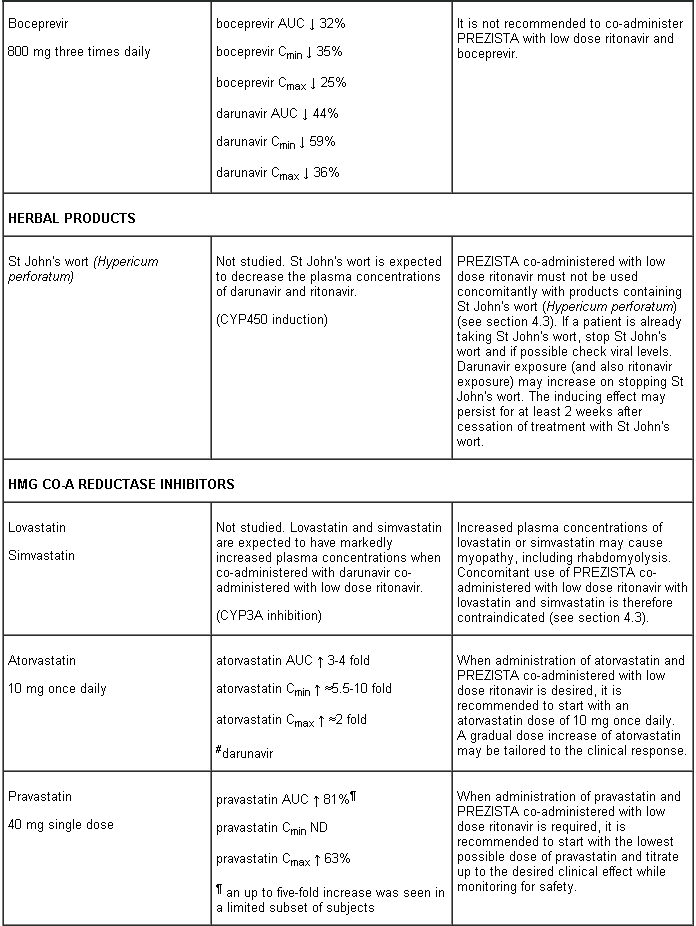
- HMG-CoA reductase inhibitors (simvastatin and lovastatin) (see section 4.5).
Special warnings and precautions for use
Patients should be advised that current antiretroviral therapy does not cure HIV and has not been proven to prevent the transmission of HIV to others through blood or sexual contact. Appropriate precautions should continue to be employed.
Regular assessment of virological response is advised. In the setting of lack or loss of virological response, resistance testing should be performed.
PREZISTA should only be used in combination with low dose ritonavir as a pharmacokinetic enhancer (see section 5.2).
Increasing the dose of ritonavir from that recommended in section 4.2 did not significantly affect darunavir concentrations and is not recommended.
Darunavir binds predominantly to α1-acid glycoprotein. This protein binding is concentration-dependent indicative for saturation of binding. Therefore, protein displacement of medicinal products highly bound to α1-acid glycoprotein cannot be ruled out (see section 4.5).
ART-experienced patients – once daily dosing
PREZISTA/rtv 800/100 mg once daily in ART-experienced patients should not be used in patients with one or more darunavir resistance associated mutations (DRV-RAMs) or HIV-1 RNA ≥ 100,000 copies/ml or CD4+ cell count < 100 cells x 106/l (see section 4.2). The efficacy and safety of PREZISTA/rtv 800/100 mg once daily in combination with optimised background regimen (OBR) for the treatment of HIV-1 infection in ART-experienced adults with no darunavir resistance associated mutations (DRV-RAMs) was evaluated in one trial with a duration of 48 weeks. Combinations with OBRs other than ≥ 2 NRTIs have not been studied in this population. Limited data are available in patients with HIV-1 clades other than B (see section 5.1).
Paediatric population
PREZISTA is not recommended for use in paediatric patients below 3 years of age or less than 15 kg body weight (see sections 4.2 and 5.3).
Elderly
As limited information is available on the use of PREZISTA in patients aged 65 and over, caution should be exercised in the administration of PREZISTA in elderly patients, reflecting the greater frequency of decreased hepatic function and of concomitant disease or other therapy (see sections 4.2 and 5.2).
Severe skin reactions
During the clinical development program (N=3,063), severe skin reactions, which may be accompanied with fever and/or elevations of transaminases, have been reported in 0.4% of patients. Stevens-Johnson Syndrome has been rarely (< 0.1%) reported, and during post-marketing experience toxic epidermal necrolysis and acute generalised exanthematous pustulosis have been reported. Discontinue PREZISTA/rtv immediately if signs or symptoms of severe skin reactions develop. These can include but are not limited to severe rash or rash accompanied by fever, general malaise, fatigue, muscle or joint aches, blisters, oral lesions, conjunctivitis, hepatitis and/or eosinophilia.
Rash occurred more commonly in treatment-experienced patients receiving regimens containing PREZISTA + raltegravir compared to patients receiving PREZISTA without raltegravir or raltegravir without PREZISTA (see section 4.8).
Darunavir contains a sulphonamide moiety. PREZISTA should be used with caution in patients with a known sulphonamide allergy.
Hepatotoxicity
Drug-induced hepatitis (e.g. acute hepatitis, cytolytic hepatitis) has been reported with PREZISTA/rtv. During the clinical development program (N=3,063), hepatitis was reported in 0.5% of patients receiving combination antiretroviral therapy with PREZISTA/rtv. Patients with pre-existing liver dysfunction, including chronic active hepatitis B or C, have an increased risk for liver function abnormalities including severe and potentially fatal hepatic adverse events. In case of concomitant antiviral therapy for hepatitis B or C, please refer to the relevant product information for these medicinal products.
Appropriate laboratory testing should be conducted prior to initiating therapy with PREZISTA/rtv and patients should be monitored during treatment. Increased AST/ALT monitoring should be considered in patients with underlying chronic hepatitis, cirrhosis, or in patients who have pre-treatment elevations of transaminases, especially during the first several months of PREZISTA/rtv treatment.
If there is evidence of new or worsening liver dysfunction (including clinically significant elevation of liver enzymes and/or symptoms such as fatigue, anorexia, nausea, jaundice, dark urine, liver tenderness, hepatomegaly) in patients using PREZISTA/rtv, interruption or discontinuation of treatment should be considered promptly.
Patients with coexisting conditions
Hepatic impairment: The safety and efficacy of PREZISTA have not been established in patients with severe underlying liver disorders and PREZISTA is therefore contraindicated in patients with severe hepatic impairment. Due to an increase in the unbound darunavir plasma concentrations, PREZISTA should be used with caution in patients with mild or moderate hepatic impairment (see sections 4.2, 4.3 and 5.2).
Renal impairment: No special precautions or dose adjustments are required in patients with renal impairment. As darunavir and ritonavir are highly bound to plasma proteins, it is unlikely that they will be significantly removed by haemodialysis or peritoneal dialysis. Therefore, no special precautions or dose adjustments are required in these patients (see sections 4.2 and 5.2).
Haemophiliac patients: There have been reports of increased bleeding, including spontaneous skin haematomas and haemarthrosis in patients with haemophilia type A and B treated with PIs. In some patients additional factor VIII was given. In more than half of the reported cases, treatment with PIs was continued or reintroduced if treatment had been discontinued. A causal relationship has been suggested, although the mechanism of action has not been elucidated. Haemophiliac patients should therefore be made aware of the possibility of increased bleeding.
Diabetes mellitus/hyperglycaemia
New onset diabetes mellitus, hyperglycaemia, or exacerbation of existing diabetes mellitus has been reported in patients receiving antiretroviral therapy, including PIs. In some of these patients the hyperglycaemia was severe and in some cases also associated with ketoacidosis. Many patients had confounding medical conditions some of which required therapy with agents that have been associated with the development of diabetes mellitus or hyperglycaemia.
Fat redistribution and metabolic disorders
Combination antiretroviral therapy has been associated with redistribution of body fat (lipodystrophy) in HIV infected patients. The long-term consequences of these events are currently unknown. Knowledge about the mechanism is incomplete. A connection between visceral lipomatosis and PIs and lipoatrophy and NRTIs has been hypothesised. A higher risk of lipodystrophy has been associated with individual factors such as older age and with drug related factors such as longer duration of antiretroviral treatment and associated metabolic disturbances. Clinical examination should include evaluation for physical signs of fat redistribution. Consideration should be given to measurement of fasting serum lipids and blood glucose. Lipid disorders should be managed as clinically appropriate (see section 4.8).
Osteonecrosis
Although the etiology is considered to be multifactorial (including corticosteroid use, alcohol consumption, severe immunosuppression, higher body mass index), cases of osteonecrosis have been reported particularly in patients with advanced HIV disease and/or long-term exposure to combination antiretroviral therapy (CART). Patients should be advised to seek medical advice if they experience joint aches and pain, joint stiffness or difficulty in movement.
Immune reactivation syndrome
In HIV infected patients with severe immune deficiency at the time of institution of combination antiretroviral therapy (CART), an inflammatory reaction to asymptomatic or residual opportunistic pathogens may arise and cause serious clinical conditions, or aggravation of symptoms. Typically, such reactions have been observed within the first weeks or months of initiation of CART. Relevant examples are cytomegalovirus retinitis, generalised and/or focal mycobacterial infections and pneumonia caused by Pneumocystis jirovecii (formerly known as Pneumocystis carinii). Any inflammatory symptoms should be evaluated and treatment instituted when necessary. In addition, reactivation of herpes simplex and herpes zoster has been observed in clinical studies with PREZISTA co-administered with low dose ritonavir.
Interactions with medicinal products
Several of the interaction studies have been performed at lower than recommended doses of darunavir. The effects on co-administered medicinal products may thus be underestimated and clinical monitoring of safety may be indicated. For full information on interactions with other medicinal products see section 4.5.
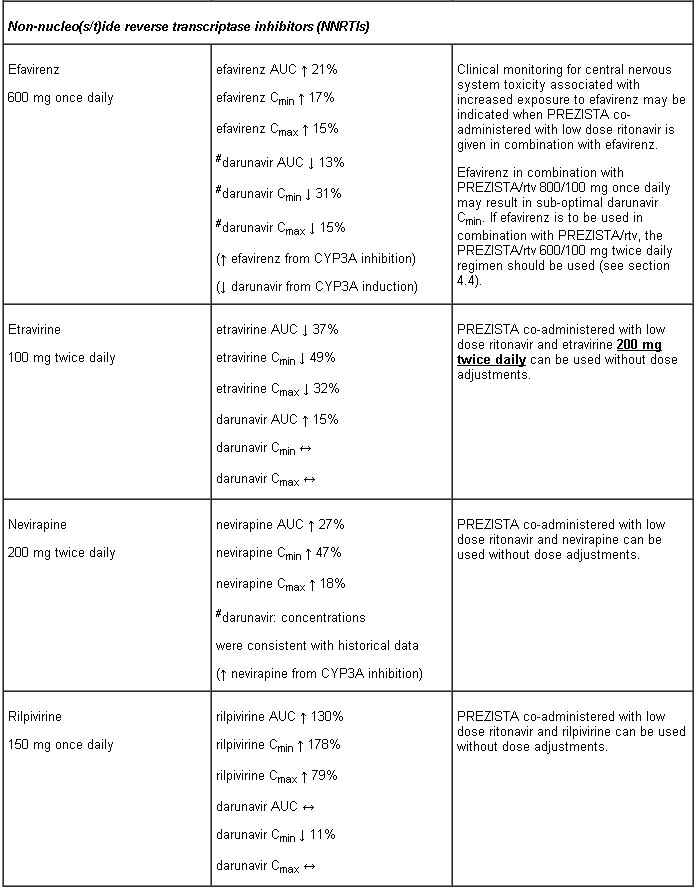
Efavirenz in combination with PREZISTA/rtv 800/100 mg once daily may result in sub-optimal darunavir Cmin. If efavirenz is to be used in combination with PREZISTA/rtv, the PREZISTA/rtv 600/100 mg twice daily regimen should be used(see section 4.5).
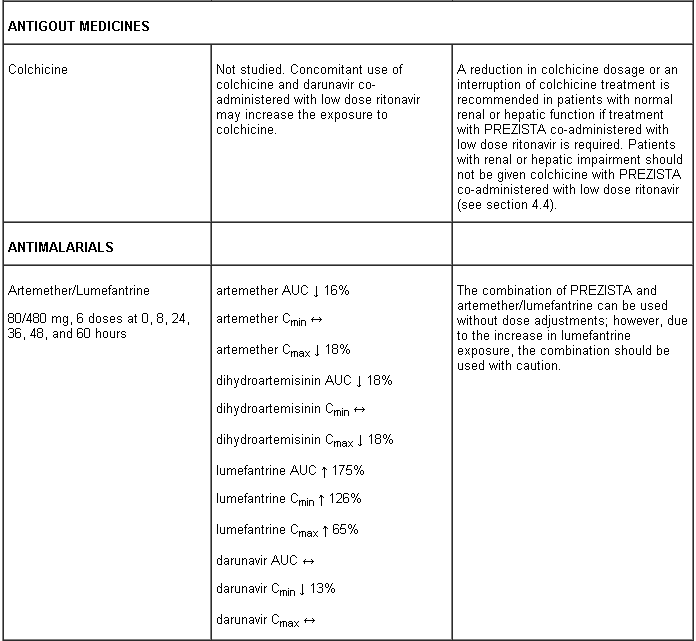
Life-threatening and fatal drug interactions have been reported in patients treated with colchicine and strong inhibitors of CYP3A and P-glycoprotein (Pgp: see section 4.5).
PREZISTA tablets (400mg and 600mg) contain sunset yellow FCF (E110) which may cause an allergic reaction.
Interaction with other medicinal products and other forms of interaction
Interaction studies have only been performed in adults.
Darunavir and ritonavir are both inhibitors of the CYP3A isoform. Co-administration of darunavir and ritonavir and medicinal products primarily metabolised by CYP3A may result in increased systemic exposure to such medicinal products, which could increase or prolong their therapeutic effect and adverse reactions.
PREZISTA co-administered with low dose ritonavir must not be combined with medicinal products that are highly dependent on CYP3A for clearance and for which increased systemic exposure is associated with serious and/or life-threatening events (narrow therapeutic index). These medicinal products include amiodarone, bepridil, quinidine, systemic lidocaine, astemizole, alfuzosin, terfenadine, sildenafil (when used for the treatment of pulmonary arterial hypertension), midazolam administered orally, triazolam, cisapride, pimozide, sertindole, simvastatin, lovastatin and the ergot alkaloids (e.g. ergotamine, dihydroergotamine, ergonovine and methylergonovine) (see section 4.3).
The overall pharmacokinetic enhancement effect by ritonavir was an approximate 14-fold increase in the systemic exposure of darunavir when a single dose of 600 mg darunavir was given orally in combination with ritonavir at 100 mg twice daily. Therefore, PREZISTA must only be used in combination with low dose ritonavir as a pharmacokinetic enhancer (see sections 4.4 and 5.2).
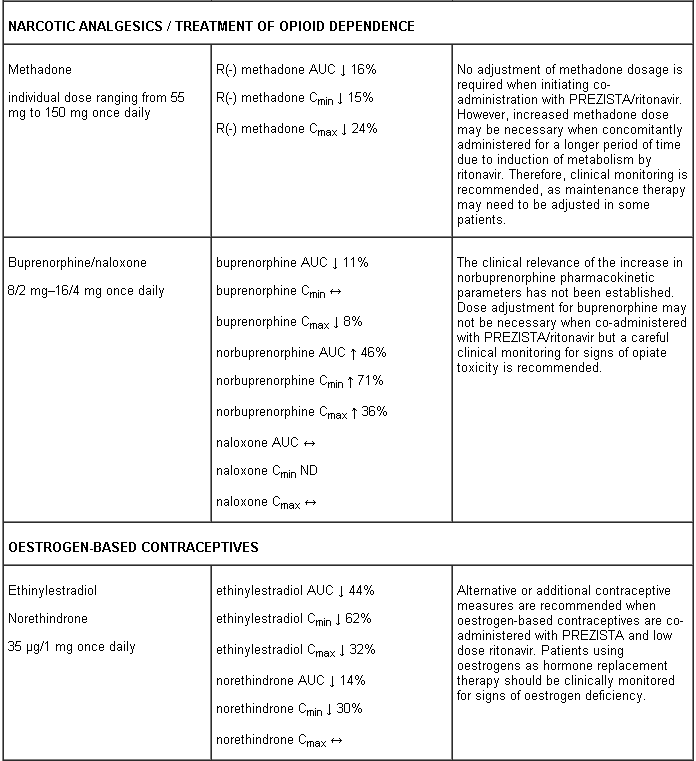
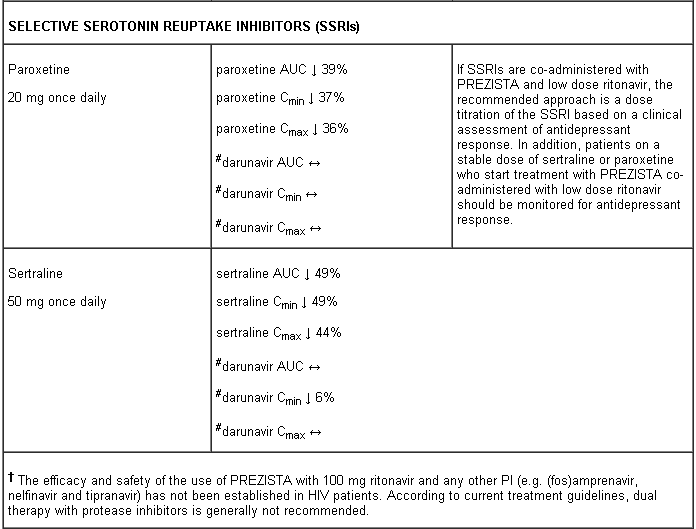
A clinical study utilising a cocktail of medicinal products that are metabolised by cytochromes CYP2C9, CYP2C19 and CYP2D6 demonstrated an increase in CYP2C9 and CYP2C19 activity and inhibition of CYP2D6 activity in the presence of PREZISTA/rtv, which may be attributed to the presence of low dose ritonavir. Co-administration of darunavir and ritonavir and medicinal products which are primarily metabolised by CYP2D6 (such as flecainide, propafenone, metoprolol) may result in increased plasma concentrations of these medicinal products, which could increase or prolong their therapeutic effect and adverse reactions. Co-administration of darunavir and ritonavir and medicinal products primarily metabolised by CYP2C9 (such as warfarin) and CYP2C19 (such as methadone) may result in decreased systemic exposure to such medicinal products, which could decrease or shorten their therapeutic effect.
Although the effect on CYP2C8 has only been studied in vitro, co-administration of darunavir and ritonavir and medicinal products primarily metabolised by CYP2C8 (such as paclitaxel, rosiglitazone, repaglinide) may result in decreased systemic exposure to such medicinal products, which could decrease or shorten their therapeutic effect.
Medicinal products that affect darunavir/ritonavir exposure
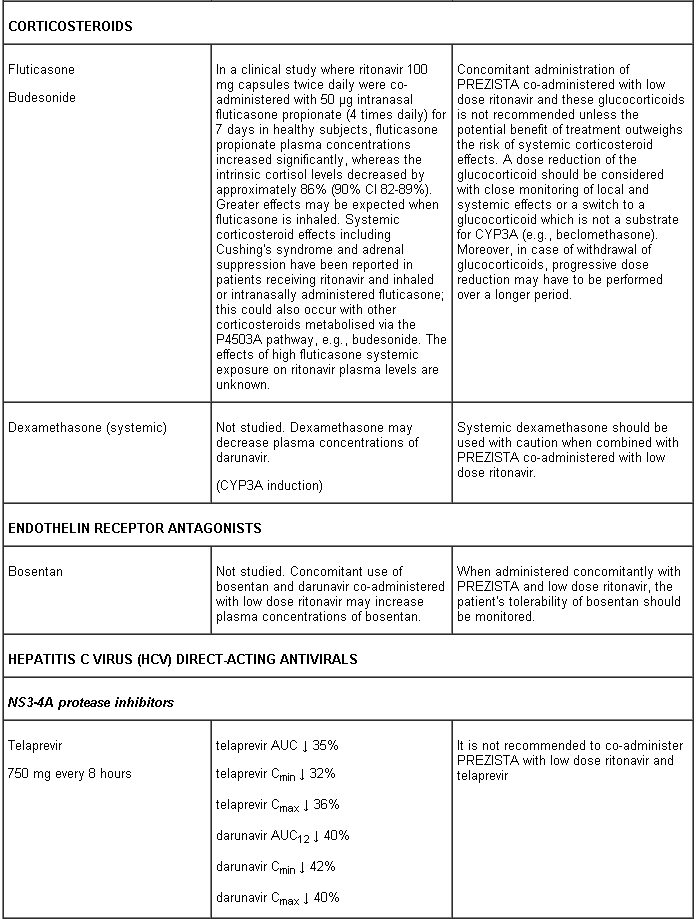
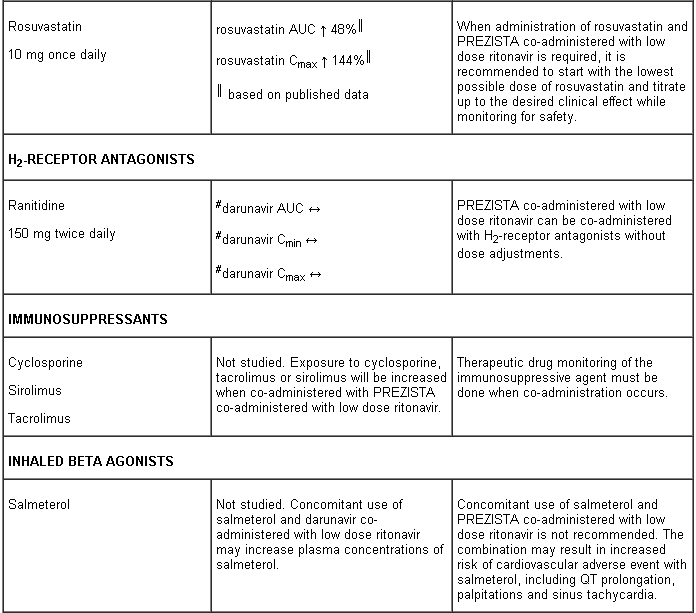
Darunavir and ritonavir are metabolised by CYP3A. Medicinal products that induce CYP3A activity would be expected to increase the clearance of darunavir and ritonavir, resulting in lowered plasma concentrations of darunavir and ritonavir (e.g. rifampicin, St John’s wort, lopinavir). Co-administration of darunavir and ritonavir and other medicinal products that inhibit CYP3A may decrease the clearance of darunavir and ritonavir and may result in increased plasma concentrations of darunavir and ritonavir (e.g. indinavir, systemic azoles like ketoconazole and clotrimazole). These interactions are described in the interaction table below.
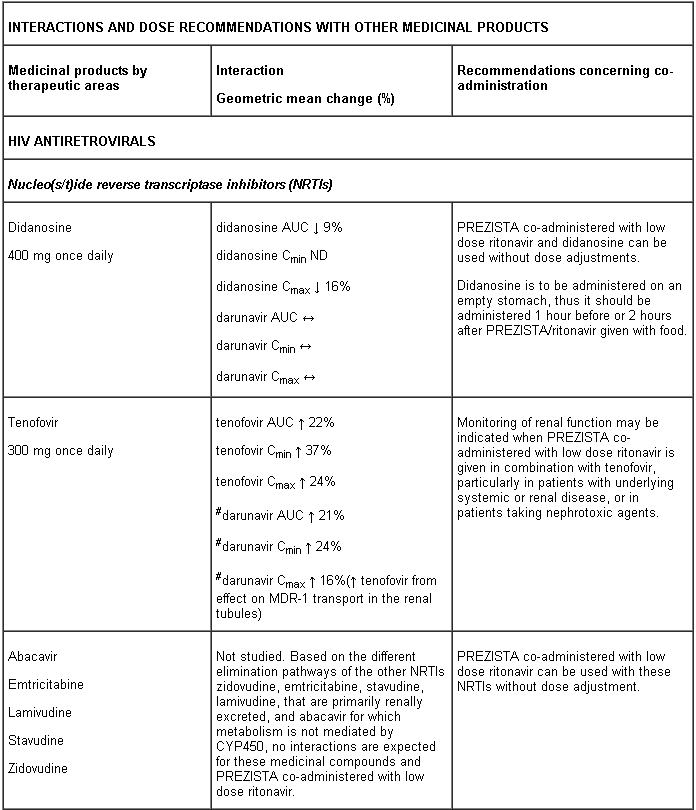
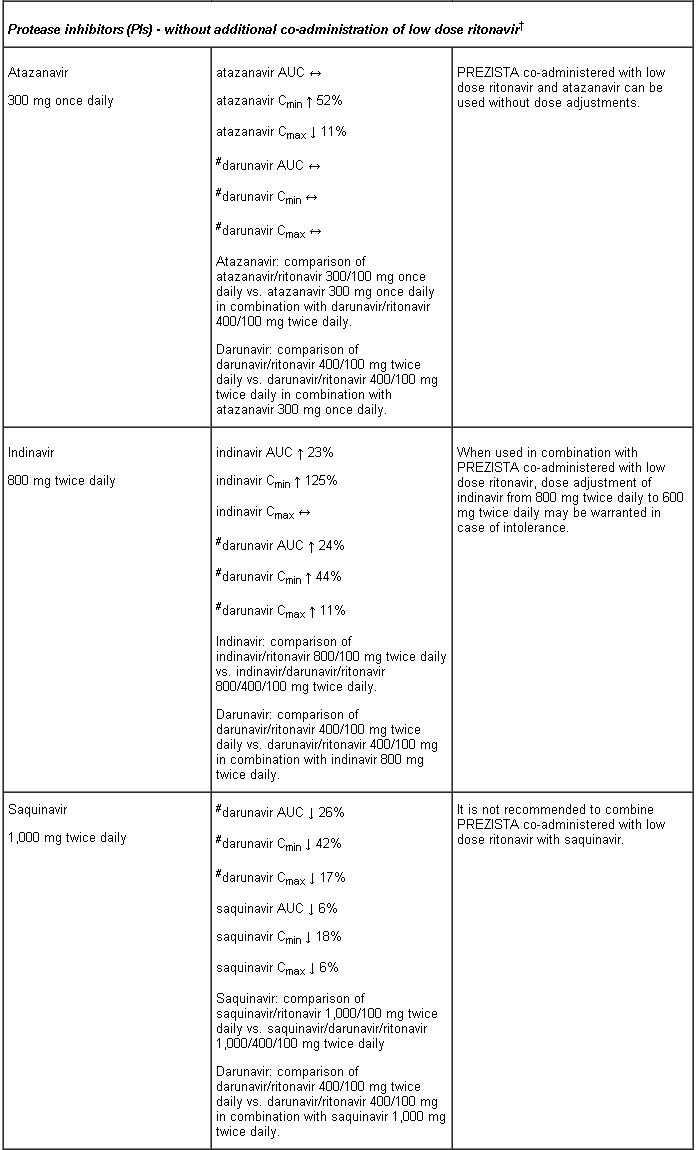
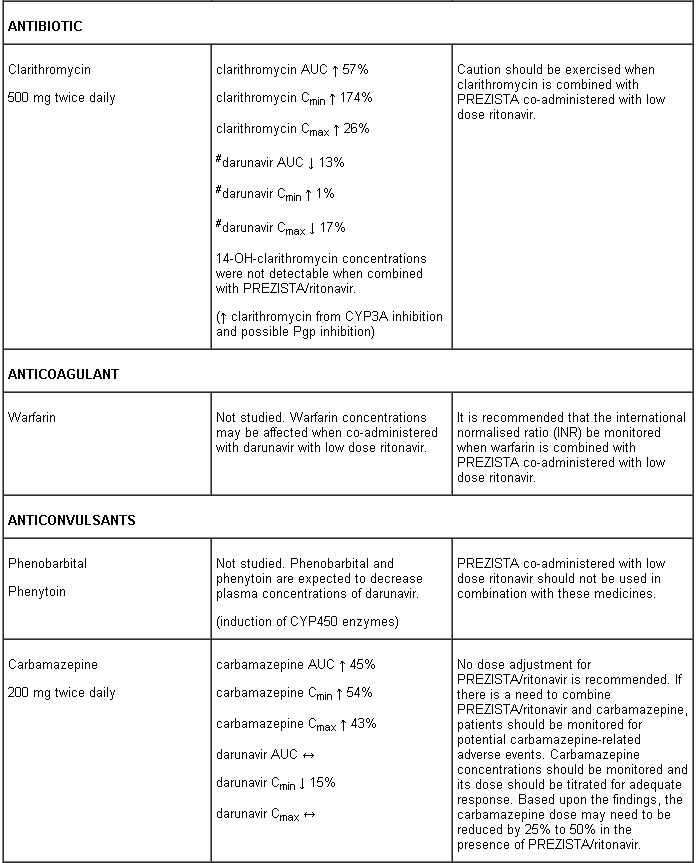
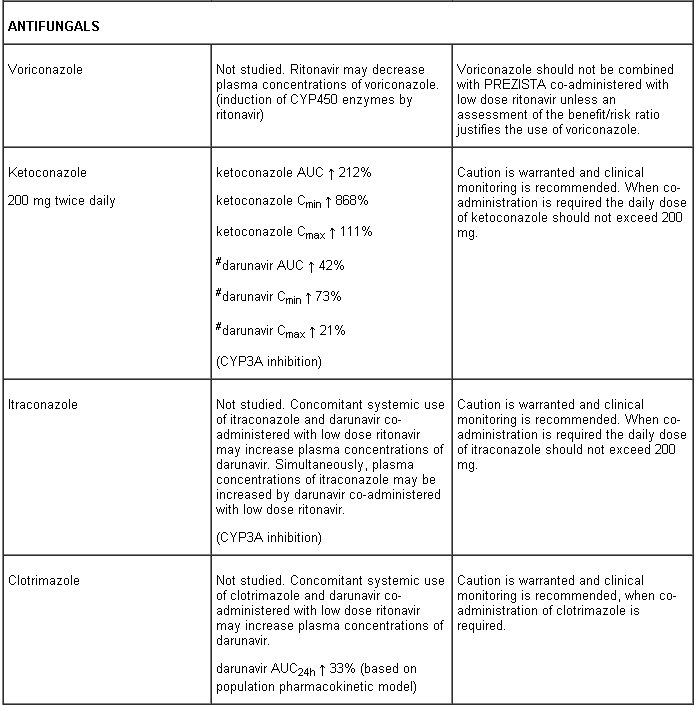
Interaction table
Interactions between darunavir/ritonavir and antiretroviral and non-antiretroviral medicinal products are listed in the table below (not determined as “ND”). The direction of the arrow for each pharmacokinetic parameter is based on the 90% confidence interval of the geometric mean ratio being within (↔), below (↓) or above (↑) the 80-125% range.
Several of the interaction studies (indicated by # in the table below) have been performed at lower than recommended doses of darunavir or with a different dosing regimen (see section 4.2 Posology). The effects on co-administered medicinal products may thus be underestimated and clinical monitoring of safety may be indicated.
Fertility, pregnancy and lactation
Pregnancy
As a general rule, when deciding to use antiretroviral agents for the treatment of HIV infection in pregnant women and consequently for reducing the risk of HIV vertical transmission to the newborn, the animal data as well as the clinical experience in pregnant women should be taken into account.
There are no adequate and well controlled studies with darunavir in pregnant women. Studies in animals do not indicate direct harmful effects with respect to pregnancy, embryonal/foetal development, parturition or postnatal development (see section 5.3).
PREZISTA co-administered with low dose ritonavir should be used during pregnancy only if the potential benefit justifies the potential risk.
Breast-feeding
It is not known whether darunavir is excreted in human milk. Studies in rats have demonstrated that darunavir is excreted in milk and at high levels (1,000 mg/kg/day) resulted in toxicity. Because of both the potential for HIV transmission and the potential for adverse reactions in breast-fed infants, mothers should be instructed not to breast-feed under any circumstances if they are receiving PREZISTA.
Fertility
No human data on the effect of darunavir on fertility are available. There was no effect on mating or fertility with darunavir treatment in rats (see section 5.3).
Effects on ability to drive and use machines
PREZISTA in combination with ritonavir has no or negligible influence on the ability to drive and use machines. However, dizziness has been reported in some patients during treatment with regimens containing PREZISTA co-administered with low dose ritonavir and should be borne in mind when considering a patient’s ability to drive or operate machinery (see section 4.8).
Undesirable effects
Summary of the safety profile
During the clinical development program (N=1,968 treatment-experienced subjects who initiated therapy with PREZISTA/rtv 600/100 mg twice daily), 49.5% of subjects experienced at least one adverse reaction. The total mean treatment duration for subjects was 48.58 weeks. The most frequent adverse reactions reported in clinical trials and as spontaneous reports are diarrhoea, immune reconstitution syndrome, nausea, pyrexia and rash. The most frequent serious reactions are diarrhoea, hepatitis, immune reconstitution syndrome, pyrexia and rash.
In the 96 week analysis, the safety profile of PREZISTA/rtv 800/100 mg once daily in treatment-naïve subjects was similar to that seen with PREZISTA/rtv 600/100 mg twice daily in treatment-experienced subjects except for nausea which was observed more frequently in treatment-naïve subjects. This was driven by mild intensity nausea. No new safety findings were identified in the 192 week analysis of the treatment-naive subjects in which the mean treatment duration of PREZISTA/rtv 800/100 mg once daily was 162.5 weeks.
Tabulated list of adverse reactions
Adverse reactions are listed by system organ class (SOC) and frequency category. Within each frequency category, adverse reactions are presented in order of decreasing seriousness. Frequency categories are defined as follows: very common (≥ 1/10), common (≥ 1/100 to < 1/10), uncommon (≥ 1/1,000 to < 1/100), rare (≥ 1/10,000 to < 1/1,000) and not known (frequency cannot be estimated from the available data).
Adverse reactions in clinical trials and post-marketing:
Infections and infestations
Uncommon: herpes simplex
Blood and lymphatic system disorders
Uncommon: thrombocytopenia, neutropenia, anaemia, increased eosinophil count, leukopenia
Immune system disorders
Uncommon: immune reconstitution syndrome, (drug) hypersensitivity
Endocrine disorders
Uncommon: hypothyroidism, increased blood thyroid stimulating hormone
Metabolism and nutrition disorders
Common: lipodystrophy (including lipohypertrophy, lipodystrophy, lipoatrophy) hypertriglyceridaemia hypercholesterolaemia, hyperlipidaemia
Uncommon: diabetes mellitus, gout, anorexia, decreased appetite, decreased weight, increased weight, hyperglycaemia, insulin resistance, decreased high density lipoprotein, increased appetite, polydipsia, increased blood lactate dehydrogenase
Psychiatric disorders
Common: insomnia
Uncommon: depression, confusional state, disorientation, anxiety, altered mood, sleep disorder, abnormal dreams, nightmare, decreased libido, restlessness
Nervous system disorders
Common: headache, peripheral neuropathy, dizziness
Uncommon: syncope, convulsion, lethargy, paraesthesia, hypoaesthesia, ageusia, dysgeusia, disturbance in attention, memory impairment, somnolence, sleep phase rhythm disturbance
Eye disorders
Uncommon: visual disturbance, conjunctival hyperaemia, dry eye
Ear and labyrinth disorders
Uncommon: vertigo
Cardiac disorders
Uncommon: acute myocardial infarction, myocardial infarction, angina pectoris, prolonged electrocardiogram QT, sinus bradycardia, tachycardia, palpitations
Vascular disorders
Uncommon: hypertension, flushing
Respiratory, thoracic and mediastinal disorders
Uncommon: dyspnoea, cough, epistaxis, rhinorrhoea, throat irritation
Gastrointestinal disorders
Very common: diarrhoea
Common: vomiting, nausea, abdominal pain, increased blood amylase, dyspepsia, abdominal distension, flatulence
Uncommon: pancreatitis, gastritis, gastrooesophageal reflux disease, aphthous stomatitis, stomatitis, retching, haematemesis, dry mouth, abdominal discomfort, constipation, increased lipase, eructation, oral dysaesthesia, cheilitis, dry lip, coated tongue
Hepatobiliary disorders
Common: increased alanine aminotransferase, increased aspartate aminotransferase
Uncommon: hepatitis, cytolytic hepatitis, hepatic steatosis, hepatomegaly, increased transaminase, increased blood bilirubin, increased blood alkaline phosphatase, increased gamma-glutamyltransferase
Skin and subcutaneous tissue disorders
Common: rash (including macular, maculopapular, papular, erythematous and pruritic rash) , pruritus
Uncommon: angioedema, generalised rash, allergic dermatitis, urticaria, dermatitis, eczema, erythema, hyperhidrosis, night sweats, alopecia, acne, seborrhoeic dermatitis, skin lesion, xeroderma, dry skin, nail pigmentation
Rare: erythema multiforme, Stevens-Johnson syndrome1
Not known: toxic epidermal necrolysis, acute generalised exanthematous pustulosis
Musculoskeletal and connective tissue disorders
Uncommon: myalgia, osteonecrosis, muscle spasms, muscular weakness, musculoskeletal stiffness, arthritis, arthralgia, joint stiffness, pain in extremity, osteoporosis, increased blood creatine phosphokinase
Renal and urinary disorders
Uncommon: acute renal failure, renal failure, nephrolithiasis, increased blood creatinine, decreased creatinine renal clearance, proteinuria, bilirubinuria, dysuria, nocturia, pollakiuria
Reproductive system and breast disorders
Uncommon: erectile dysfunction, gynaecomastia
General disorders and administration site conditions
Common: asthenia, fatigue
Uncommon: pyrexia, chest pain, peripheral oedema, malaise, chills, abnormal feeling, feeling hot, irritability, pain, xerosis
Description of selected adverse reactions
Rash
In clinical trials, rash was mostly mild to moderate, often occurring within the first four weeks of treatment and resolving with continued dosing. In cases of severe skin reaction see the warning in section 4.4.
During the clinical development program of raltegravir in treatment-experienced patients, rash, irrespective of causality, was more commonly observed with regimens containing PREZISTA + raltegravir compared to those containing PREZISTA without raltegravir or raltegravir without PREZISTA. Rash considered by the investigator to be drug-related occurred at similar rates. The exposure-adjusted rates of rash (all causality) were 10.9, 4.2, and 3.8 per 100 patient-years (PYR), respectively; and for drug-related rash were 2.4, 1.1, and 2.3 per 100 PYR, respectively. The rashes observed in clinical studies were mild to moderate in severity and did not result in discontinuation of therapy (see section 4.4).
Lipodystrophy
Combination antiretroviral therapy has been associated with redistribution of body fat (lipodystrophy) in HIV patients, including loss of peripheral and facial subcutaneous fat, increased intra-abdominal and visceral fat, breast hypertrophy and dorsocervical fat accumulation (buffalo hump) (see section 4.4).
Metabolic abnormalities
Combination antiretroviral therapy has also been associated with metabolic abnormalities such as hypertriglyceridaemia, hypercholesterolaemia, insulin resistance, hyperglycaemia and hyperlactataemia (see section 4.4).
Musculoskeletal abnormalities
Increased CPK, myalgia, myositis and rarely, rhabdomyolysis have been reported with the use of protease inhibitors, particularly in combination with NRTIs.
Cases of osteonecrosis have been reported, particularly in patients with generally acknowledged risk factors, advanced HIV disease or long-term exposure to combination antiretroviral therapy (CART). The frequency of this is unknown (see section 4.4).
Immune reconstitution syndrome
In HIV infected patients with severe immune deficiency at the time of initiation of combination antiretroviral therapy, an inflammatory reaction to asymptomatic or residual opportunistic infections may arise (see section 4.4).
Bleeding in haemophiliac patients
There have been reports of increased spontaneous bleeding in haemophiliac patients receiving antiretroviral protease inhibitors (see section 4.4).
Paediatric population
The safety assessment in paediatric patients is based on the 48-week analysis of safety data from two Phase II trials. DELPHI evaluated 80 ART-experienced HIV-1 infected paediatric patients aged from 6 to 17 years and weighing at least 20 kg who received PREZISTA tablets with low dose ritonavir in combination with other antiretroviral agents. ARIEL evaluated 21 ART-experienced HIV-1 infected paediatric patients aged from 3 to < 6 years and weighing 10 kg to < 20 kg (16 participants from 15 kg to < 20 kg) who received PREZISTA oral suspension with low dose ritonavir in combination with other antiretroviral agents (see section 5.1).
Overall, the safety profile in these paediatric patients was similar to that observed in the adult population.
Other special populations
Patients co-infected with hepatitis B and/or hepatitis C virus
Among 1,968 treatment-experienced patients receiving PREZISTA co-administered with ritonavir 600/100 mg twice daily, 236 patients were co-infected with hepatitis B or C. Co-infected patients were more likely to have baseline and treatment emergent hepatic transaminase elevations than those without chronic viral hepatitis (see section 4.4).
Incompatibilities
Not applicable.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.