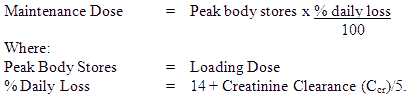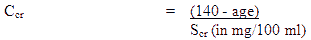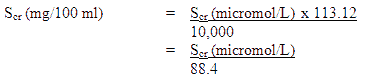Lanoxin 125 Tablets Ref.[2746] Active ingredients: Digoxin
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2012 Publisher: Aspen Pharma Trading Limited 12/13 Exchange Place I.F.S.C Dublin 1 Ireland
Therapeutic indications
Lanoxin is indicated in the management of chronic cardiac failure where the dominant problem is systolic dysfunction. Its therapeutic benefit is greatest in those patients with ventricular dilatation.
Lanoxin is specifically indicated where cardiac failure is accompanied by atrial fibrillation.
Lanoxin is indicated in the management of certain supraventricular arrhthmias, particularly chronic atrial flutter and fibrillation.
Posology and method of administration
The dose of Lanoxin for each patient has to be tailored individually according to age, lean body weight and renal function. Suggested doses are intended only as an initial guide.
The difference in bioavailability between injectable Lanoxin and oral formulations must be considered when changing from one dosage form to another. For example, if patients are switched from oral to the i.v. formulation the dosage should be reduced by approximately 33 %.
Adults with chronic cardiac failure in the absence of supraventricular arrhythmia
No loading dose is required. The usual daily dose is 125 to 250 micrograms (0.125 to 0.25 mg) for patients with normal renal function. A lower dose of 62.5 micrograms (0.0625 mg) should be considered in the elderly.
For the management of atrial fibrillation or flutter in adults and children over 10 years
Rapid Oral Loading:
If medically appropriate, rapid digitalisation may be achieved in a number of ways, such as the following:
750 to 1500 micrograms (0.75 to 1.5 mg) as a single dose.
Where there is less urgency, or greater risk of toxicity eg. in the elderly, the oral loading dose should be given in divided doses 6 hours apart, assessing clinical response before giving each additional dose. (See Special Warnings and Precautions for Use).
Slow Oral Loading:
Digitalisation may be achieved more slowly with doses of 250 to 750 micrograms (0.25 to 0.75 mg) daily for 1 week followed by an appropriate maintenance dose. A clinical response should be seen within one week.
NOTE: The choice between slow and rapid oral loading depends on the clinical state of the patient and the urgency of the condition.
Maintenance Dose:
The maintenance dosage should be based upon the percentage of the peak body stores lost each day through elimination. The following formula has had wide clinical use:
Ccr is creatinine clearance corrected to 70 kg body weight or 1.73 m² body surface area. If only serum creatinine (Scr) concentrations are available, a Ccr (Corrected to 70 kg body weight) may be estimated in men as
NOTE: Where serum creatinine values are obtained in micromol/l these may be converted to mg/100 ml (mg %) as follows
Where 113.12 is the molecular weight of creatinine.
For women, this result should be multiplied by 0.85.
NOTE: These formulae cannot be used for creatinine clearance in children.
In practice, this will mean that most patients will be maintained on 0.125 to 0.25 mg digoxin daily; however in those who show increased sensitivity to the adverse effects of digoxin, a dosage of 62.5 microgram (0.0625 mg) daily or less may suffice. Conversely, some patients may require a higher dose.
Neonates, infants and children up to 10 years of age (if cardiac glycosides have not been given in the preceding two weeks)
In the newborn, particularly in the premature infant, renal clearance of digoxin is diminished and suitable dose reductions must be observed, over and above general dosage instructions.
Beyond the immediate newborn period, children generally require proportionally larger doses than adults on the basis of body weight or body surface area, as indicated in the schedule below. Children over 10 years of age require adult dosages in proportion to their body weight.
Oral Loading Dose:
This should be administered in accordance with the following schedule:
Preterm neonates < 1.5 kg: 25 microgram/kg over 24 hours
Preterm neonates 1.5 to 2.5 kg: 30 microgram/kg over 24 hours
Term neonates to 2 years: 45 microgram/kg over 24 hours
2 to 5 years: 35 microgram/kg over 24 hours
5 to 10 years: 25 microgram/kg over 24 hours
The loading dose should be administered in divided doses with approximately half the total dose given as the first dose and further fractions of the total dose given at intervals of 4 to 8 hours, assessing clinical response before giving each additional dose.
Maintenance Dose:
The maintenance dose should be administered in accordance with the following schedule:
Preterm neonates: daily dose = 20% of 24-hour loading dose (intravenous or oral)
Term neonates and children up to 10 years: daily dose = 25% of 24-hour loading dose (intravenous or oral)
These dosage schedules are meant as guidelines and careful clinical observation and monitoring of serum digoxin levels (see Monitoring) should be used as a basis for adjustment of dosage in these paediatric patients groups.
If cardiac glycosides have been given in the two weeks preceding commencement of Lanoxin therapy, it should be anticipated that optimum loading doses of Lanoxin will be less than those recommended above.
Use in the elderly
The tendency to impaired renal function and low lean body mass in the elderly influences the pharmacokinetic of Lanoxin such that high serum digoxin levels and associated toxicity can occur quite readily, unless doses of Lanoxin lower than those in non-elderly patients are used. Serum digoxin levels should be checked regularly and hypokalaemia avoided.
Dose recommendations in renal disorder or with diuretic therapy
See Special Warnings and Precautions for Use.
Monitoring
Serum concentrations of digoxin may be expressed in conventional units of nanogram/ml (ng/ml) or SI units of nanomol/L (nmol/L). To convert ng/ml to nmol/L, multiply ng/ml by 1.28.
The serum concentration of digoxin can be determined by radioimmunoassay. Blood should be taken 6 hours or more after the last dose of Lanoxin. Several post hoc analyses of heart failure patients in the Digitalis Investigation Group trial suggest that the optimal trough digoxin serum level may be 0.5 ng/mL (0.64 nanomol/L) to 1.0 ng/mL (1.28 nanomol/L).
Digoxin toxicity is more commonly associated with serum digoxin concentration greater than 2 ng/mL. However, toxicity may occur with lower digoxin serum concentrations. In deciding whether a patient's symptoms are due to digoxin, the patients clinical state together with the serum potassium level and thyroid function are important factors.
Other glycosides, including metabolites of digoxin, can interfere with the assays that are available and one should always be wary of values which do not seem commensurate with the clinical state of the patient.
Overdose
The symptoms and signs of toxicity are generally similar to those described in the Undesirable Effects section but may be more frequent and can be more severe.
Signs and symptoms of digoxin toxicity become more frequent with levels above 2.0 nanograms/mL (2.56 nanomol/L) although there is considerable interindividual variation. However, in deciding whether a patient's symptoms are due to digoxin, the clinical state, together with serum electrolyte levels and thyroid function are important factors (see Dosage and Administration).
Adults
In adults without heart disease, clinical observation suggests that an overdose of digoxin of 10 to 15 mg was the dose resulting in death of half of the patients.
Cardiac manifestations
Cardiac manifestations are the most frequent and serious sign of both acute and chronic toxicity. Peak cardiac effects generally occur 3 to 6 hours following overdosage and may persist for the ensuing 24 hours or longer. Digoxin toxicity may result in almost any type of arrhythmia. Multiple rhythm disturbances in the same patient are common. These include paroxysmal atrial tachycardia with variable atrioventricular (AV) block, accelerated junctional rhythm, slow atrial fibrillation (with very little variation in the ventricular rate) and bi directional ventricular tachycardia.
Premature ventricular contractions (PVCs) are often the earliest and most common arrhythmia. Bigeminy or trigeminy also occur frequently.
Sinus bradycardia and other bradyarrhythmias are very common.
First, second, third degree heart blocks and AV disocciation are also common.
Early toxicity may only be manifested by prolongation of the PR interval.
Ventricular tachycardia may also be a manifestation of toxicity.
Cardiac arrest from asystole or ventricular fibrillation due to digoxin toxicity is usually fatal.
Hypokalaemia may contribute to toxicity (see Warnings and Precautions).
Non-cardiac manifestations
Acute massive digoxin overdosage can result in mild to pronounced hyperkalaemia due to inhibition of the sodium-potassium (Na+-K+) pump.
Gastrointestinal symptoms are very common in both acute and chronic toxicity. The symptoms precede cardiac manifestations in approximately half of the patients in most literature reports. Anorexia, nausea and vomiting have been reported with an incidence up to 80%. These symptoms usually present early in the course of an overdose.
Neurologic and visual manifestations occur in both acute and chronic toxicity. Dizziness, various CNS disturbances, fatigue and malaise are very common. The most frequent visual disturbance is an aberration of colour vision (predominance of yellow green). These neurological and visual symptoms may persist even after other signs of toxicity have resolved.
In chronic toxicity, non-specific extracardiac symptoms, such as malaise and weakness, may predominate.
Children
In children aged 1 to 3 years without heart disease, clinical observation suggests that an overdose of digoxin of 6 to 10 mg was the dose resulting in death in half of the patients.
Most manifestations of toxicity in children occur during or shortly after the loading phase with digoxin.
Cardiac manifestations
The same arrhythmias or combination of arrhythmias that occur in adults can occur in children. Sinus tachycardia, supraventricular tachycardia, and rapid atrial fibrillation are seen less frequently in the paediatric population.
Paediatric patients are more likely to present with an AV conduction disturbance or a sinus bradycardia.
Ventricular ectopy is less common, however in massive overdose, ventricular ectopy, ventricular tachycardia and ventricular fibrillation have been reported.
Any arrhythmia or alteration in cardiac conduction that develops in a child taking digoxin should be assumed to be caused by digoxin, until further evaluation proves otherwise.
Extracardiac manifestations
The frequent extracardiac manifestations similar to those seen in adults are gastrointestinal, CNS and visual. However, nausea and vomiting are not frequent in infants and small children.
In addition to the undesirable effects seen with recommended doses, weight loss in older age groups and failure to thrive in infants, abdominal pain due to mesenteric artery ischaemia, drowsiness and behavioural disturbances including psychotic manifestations have been reported in overdose.
Treatment
After recent ingestion, such as accidental or deliberate self-poisoning, the load available for absorption may be reduced by gastric lavage.
Patients with massive digitalis ingestion should receive large doses of activated charcoal to prevent absorption and bind digoxin in the gut during enteroenteric recirculation.
If more than 25 mg of digoxin was ingested by an adult without heart disease, death or progressive toxicity responsive only to digoxin-binding Fab antibody fragments (Digibind) resulted. If more that 10 mg of digoxin was ingested by a child aged 1 to 3 years without heart disease, the outcome was uniformly fatal when Fab fragment treatment was not given.
Hypokalaemia should be corrected. In cases where a large amount of Lanoxin has been ingested hyperkalaemia may be present due to release of potassium from skeletal muscle. Before administering potassium in digoxin overdose the serum potassium level must be known.
Bradyarrhythmias may respond to atropine but temporary cardiac pacing may be required. Ventricular arrhythmias may respond to lignocaine or phenytoin.
Dialysis is not particularly effective in removing digoxin from the body in potentially life-threatening toxicity.
Rapid reversal of the complications that are associated with serious poisoning by digoxin, digitoxin and related glycosides has followed intravenous administration of digoxin-specific (ovine) antibody fragments (Fab) when other therapies have failed. Digibind is the only specific treatment for digoxin toxicity.
Ημερομηνία λήξης
Amber glass bottle: 60 months.
Blister packs: 36 months.
Special precautions for storage
Store below 25°C
Nature and contents of container
Amber glass bottle with low-density polyethylene snap-fit closure
Pack sizes: 28, 50, 500 tablets
Amber glass bottle with a clic-loc child resistant closure
Pack size: 56 tablets
White opaque PVC/aluminium foil blister
Pack sizes: 30, 60, 90, 120 tablets
Not all pack sizes may be marketed.
Special precautions for disposal and other handling
Not applicable.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.