ADRIAMYCIN Solution for injection Ref.[11117] Active ingredients: Doxorubicin
Source: FDA, National Drug Code (US) Revision Year: 2019
Product description
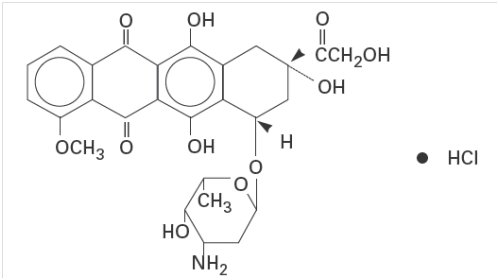
Doxorubicin is a cytotoxic anthracycline antibiotic isolated from cultures of Streptomyces peucetius var.caesius. Doxorubicin consists of a naphthacenequinone nucleus linked through a glycosidic bond at ring atom 7 to an amino sugar, daunosamine. Chemically, doxorubicin hydrochloride is (8S,10S)-10-[(3-Amino-2,3,6-trideoxy-a-L-lyxo-hexopyranosyl)-oxy]-8-glycoloyl-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-5,12-naphthacenedione hydrochloride.
The structural formula is as follows:
Doxorubicin binds to nucleic acids, presumably by specific intercalation of the planar anthracycline nucleus with the DNA double helix. The anthracycline ring is lipophilic, but the saturated end of the ring system contains abundant hydroxyl groups adjacent to the amino sugar, producing a hydrophilic center. The molecule is amphoteric, containing acidic functions in the ring phenolic groups and a basic function in the sugar amino group. It binds to cell membranes as well as plasma proteins.
It is supplied in the hydrochloride form as a sterile parenteral, isotonic solution with sodium chloride for intravenous use only.
Adriamycin (DOXOrubicin HCI) Injection, USP:
Each 2 mg/mL, 5 mL (10 mg) vial contains 10 mg Doxorubicin Hydrochloride, USP; Sodium Chloride 0.9% (to adjust tonicity) and Water for Injection q.s.; pH adjusted to 3 using Hydrochloric Acid.
Each 2 mg/mL, 10 mL (20 mg) vial contains 20 mg Doxorubicin Hydrochloride, USP; Sodium Chloride 0.9% (to adjust tonicity) and Water for Injection q.s.; pH adjusted to 3 using Hydrochloric Acid.
Each 2 mg/mL, 25 mL (50 mg) vial contains 50 mg Doxorubicin Hydrochloride, USP; Sodium Chloride 0.9% (to adjust tonicity) and Water for Injection q.s.; pH adjusted to 3 using Hydrochloric Acid.
Each 2 mg/mL, 100 mL (200 mg) multiple dose vial contains 200 mg Doxorubicin Hydrochloride, USP; Sodium Chloride 0.9% (to adjust tonicity) and Water for Injection q.s.; pH adjusted to 3 using Hydrochloric Acid.
| Dosage Forms and Strengths |
|---|
|
Adriamycin (DOXOrubicin HCl) Injection, USP: Vials contain 10 mg/5 mL, 20 mg/10 mL, 50 mg/25 mL, 150 mg/75 mL, and 200 mg/100 mL doxorubicin hydrochloride as a clear red solution. |
| How Supplied |
|---|
|
Adriamycin (DOXOrubicin HCI) Injection, USP is supplied in single-dose, flip-top vials, as a red-orange solution containing Doxorubicin Hydrochloride, USP 2 mg/mL in the following package strengths: NDC 0143-9549-01: 10 mg in 5 mL; individually boxed. NDC 0143-9548-01: 20 mg in 10 mL; individually boxed. NDC 0143-9547-01: 50 mg in 25 mL; individually boxed. Store refrigerated, 2° to 8°C (36° to 46°F). Protect from light. Retain in carton until time of use. Discard unused portion. Adriamycin (DOXOrubicin HCI) Injection, USP is supplied in a sterile, multiple dose, flip-top vial, as a red-orange solution containing Doxorubicin Hydrochloride, USP 2 mg/mL in the following package strength: NDC 0143-9546-01: 200 mg in 100 mL; individually boxed. Manufactured by: THYMOORGAN PHARMAZIE GmbH, Schiffgraben 23, 38690 Goslar, Germany Distributed by: WEST-WARD PHARMACEUTICALS, Eatontown, NJ 07724 USA |
Drugs
| Drug | Countries | |
|---|---|---|
| ADRIAMYCIN | Australia, Estonia, New Zealand, Singapore, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.