ADRIAMYCIN Solution for injection Ref.[11117] Active ingredients: Doxorubicin
Source: FDA, National Drug Code (US) Revision Year: 2019
12.1. Mechanism of Action
The cytotoxic effect of doxorubicin on malignant cells and its toxic effects on various organs are thought to be related to nucleotide base intercalation and cell membrane lipid binding activities of doxorubicin. Intercalation inhibits nucleotide replication and action of DNA and RNA polymerases. The interaction of doxorubicin with topoisomerase II to form DNA-cleavable complexes appears to be an important mechanism of doxorubicin cytocidal activity.
12.3. Pharmacokinetics
Pharmacokinetic studies conducted in patients with various types of tumors have shown that doxorubicin follows multiphasic disposition after intravenous injection. The distribution half-life is approximately 5 minutes, while the terminal half-life is 20 to 48 hours. In four patients, doxorubicin demonstrated dose-independent pharmacokinetics across a dose range of 30 to 70 mg/m².
Distribution
Steady-state distribution volume ranges from 809 to 1214 L/m². Binding of doxorubicin and its major metabolite, doxorubicinol, to plasma proteins is about 75% and is independent of plasma concentration of doxorubicin up to 1.1 mcg/mL.
Doxorubicin was measured in the milk of one lactating patient after therapy with 70 mg/m² of doxorubicin given as a 15-minute intravenous infusion. The peak milk concentration at 24 hours after treatment was 4.4-fold greater than the corresponding plasma concentration. Doxorubicin was detectable in the milk up to 72 hours.
Doxorubicin does not cross the blood brain barrier.
Metabolism
Enzymatic reduction at the 7 position and cleavage of the daunosamine sugar yields aglycones which are accompanied by free radical formation, the local production of which may contribute to the cardiotoxic activity of doxorubicin. Disposition of doxorubicinol in patients is formation rate limited, with the terminal half-life of doxorubicinol being similar to doxorubicin. The relative exposure of doxorubicinol, i.e., the ratio between the AUC of doxorubicinol and the AUC of doxorubicin is approximately 0.5.
Excretion
Plasma clearance is in the range 324 to 809 mL/min/m² and is predominately by metabolism and biliary excretion. Approximately 40% of the dose appears in the bile in 5 days, while only 5 to 12% of the drug and its metabolites appear in the urine during the same time period. In urine, <3% of the dose was recovered as doxorubicinol over 7 days.
Systemic clearance of doxorubicin is significantly reduced in obese women with ideal body weight greater than 130%. There was a significant reduction in clearance without any change in volume of distribution in obese patients when compared with normal patients with less than 115% ideal body weight.
Pediatric patients
Following administration of doses ranging from 10 to 75 mg/m² of doxorubicin to 60 children and adolescents ranging from 2 months to 20 years of age, doxorubicin clearance averaged 1443 ± 114 mL/min/m². Further analysis demonstrated that clearance in 52 children greater than 2 years of age (1540 mL/min/m²) was increased compared with adults. However, clearance in infants younger than 2 years of age (813 mL/min/m²) was decreased compared with older children and approached the range of clearance values determined in adults [see Use in Specific Populations (8.4)].
Patient Gender
There is no recommended dose adjustment based on gender. A published clinical study involving 6 men and 21 women with no prior anthracycline therapy reported a significantly higher median doxorubicin clearance in men compared to women (1088 mL/min/m² versus 433 mL/min/m²). However, the terminal half-life of doxorubicin was longer in men compared to women (54 versus 35 hours).
Patients with hepatic impairment
The clearance of doxorubicin and doxorubicinol was reduced in patients with elevation in serum bilirubin [see Dosage and Administration (2.2) and Warnings and Precautions (5.5)].
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Doxorubicin treatment results in an increased risk of secondary malignancies based on postmarketing reports [see Warnings and Precautions (5.2)]. Doxorubicin was mutagenic in the in vitro Ames assay, and clastogenic in multiple in vitro assays (CHO cell, V79 hamster cell, human lymphoblast, and SCE assays) and the in vivo mouse micronucleus assay.
Doxorubicin decreased fertility in female rats at the doses of 0.05 and 0.2 mg/kg/day (approximately 0.005 and 0.02 times the recommended human dose, based on body surface area).
A single intravenous dose of 0.1 mg/kg doxorubicin (approximately 0.01 times the recommended human dose based on body surface area) was toxic to male reproductive organs in animal studies, producing testicular atrophy, diffuse degeneration of the seminiferous tubules, and oligospermia/hypospermia in rats. Doxorubicin induces DNA damage in rabbit spermatozoa and dominant lethal mutations in mice.
14. Clinical Studies
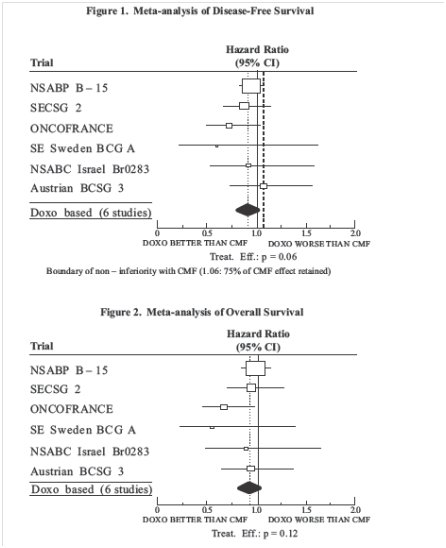
The clinical efficacy of doxorubicin containing regimens for the post-operative, adjuvant treatment of surgically resected breast cancer was evaluated in a meta-analysis conducted by the Early Breast Cancer Trialists Collaborative Group (EBCTCG). The EBCTCG meta-analyses compared cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) to no chemotherapy (19 trials including 7523 patients) and doxorubicin containing regimens with CMF as an active control (6 trials including 3510 patients). Data from the meta-analysis of trials comparing CMF to no therapy were used to establish the historical treatment effect size for CMF regimens. The major efficacy outcome measures were disease-free survival (DFS) and overall survival (OS).
Of the 3510 women (2157 received doxorubicin containing regimens and 1353 received CMF treatment) with early breast cancer involving axillary lymph nodes included in the six trials from the meta-analyses, approximately 70% were premenopausal and 30% were postmenopausal. At the time of the meta-analysis, 1745 first recurrences and 1348 deaths had occurred. The analyses demonstrated that doxorubicin containing regimens retained at least 75% of the historical CMF adjuvant effect on DFS with a hazard ratio (HR) of 0.91 (95% CI, 0.8 to 1.01) and on OS with a HR of 0.91 (95% CI, 0.8 to 1.03). Results of these analyses for both DFS and OS are provided in Table 2 and Figures 1 and 2.
Table 2. Summary of Randomized Trials Comparing Doxorubicin Containing Regimens Versus CMF in Meta-Analysis:
| Study (starting year) | Regimens | No. of Cycles | No. of Patients | Doxorubicin Containing Regimens vs. CMF HR** (95% CI) | |
|---|---|---|---|---|---|
| DFS | OS | ||||
| NSABP B-15(1984) | AC CMF | 4 6 | 1562* 776 | 0.93 (0.82 to 1.06) | 0.97 (0.83 to 1.12) |
| SECSG 2 (1976) | FAC CMF | 6 6 | 260 268 | 0.86 (0.66 to 1.13) | 0.93 (0.69 to 1.26) |
| ONCOFRANCE (1978) | FAC VCMF | 12 12 | 138 113 | 0.71 (0.49 to 1.03) | 0.65 (0.44 to 0.96) |
| SE Sweden BCG A (1980) | AC CMF | 6 6 | 21 22 | 0.59 (0.22 to 1.61) | 0.53 (0.21 to 1.37) |
| NSABC Israel Br0283 (1983) | AVbCMF† CMF | 4 6 6 | 55 50 | 0.91 (0.53 to 1.57) | 0.88 (0.47 to 1.63) |
| Austrian BCSG 3 (1984) | CMFVA CMF | 6 8 | 121 124 | 1.07 (0.73 to 1.55) | 0.93 (0.64 to 1.35) |
| Combined Studies | Doxorubicin Containing Regimens CMF | 2157 1353 | 0.91 (0.82 to 1.01) | 0.91 (0.81 to 1.03) | |
Abbreviations: DFS = disease free survival; OS = overall survival; AC = doxorubicin, cyclophosphamide; AVbCMF = doxorubicin, vinblastine, cyclophosphamide, methotrexate, 5-fluorouracil; CMF = cyclophosphamide, methotrexate, 5-fluorouracil; CMFVA = cyclophosphamide, methotrexate, 5-fluorouracil, vincristine, doxorubicin; FAC = 5-fluorouracil, doxorubicin, cyclophosphamide; FACV = 5-fluorouracil, doxorubicin, cyclophosphamide, vincristine; HR = hazard ratio; CI = confidence interval
* Includes pooled data from patients who received either AC alone for 4 cycles, or who were treated with AC for 4 cycles followed by 3 cycles of CMF.
** a hazard ratio of less than 1 indicates that the treatment with doxorubicin containing regimens is associated with lower risk of disease recurrences or death compared to the treatment with CMF.
† Patients received alternating cycles of AVb and CMF.
Figure 1. Meta-analysis of Disease-Free Survival:
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.