ANDEXXA Powder for solution for injection Ref.[9968] Active ingredients: Andexanet alfa
Source: FDA, National Drug Code (US) Revision Year: 2020
12.1. Mechanism of Action
Coagulation factor Xa (recombinant), inactivated-zhzo exerts its procoagulant effect by binding and sequestering the FXa inhibitors, rivaroxaban and apixaban. Another observed procoagulant effect of the ANDEXXA protein is its ability to bind to, and inhibit the activity of, Tissue Factor Pathway Inhibitor (TFPI). Inhibition of TFPI activity can increase tissue factor (TF)-initiated thrombin generation.
12.2. Pharmacodynamics
The effects of ANDEXXA can be measured using assays for its anti-FXa activity, free fraction of FXa inhibitor, and thrombin generation. In addition to its ability to sequester the FXa inhibitors, rivaroxaban and apixaban, ANDEXXA has been shown to inhibit TFPI activity.
The dose and dosing regimen of ANDEXXA that are required to reverse anti-FXa activity and to restore thrombin generation were determined in dose-ranging studies on healthy volunteers. Dosing of ANDEXXA, as a bolus followed by a two-hour continuous infusion, resulted in a rapid decrease in anti-FXa activity (within two minutes after the completion of the bolus administration) followed by reduced anti-FXa activity that was maintained throughout the duration of the continuous infusion [see Clinical Studies (14)]. The anti-FXa activity returned to the placebo levels approximately two hours after completion of a bolus or continuous infusion, whereas TFPI activity in plasma returned to the pretreatment levels approximately 96 hours following ANDEXXA administration.
Elevation of TF-initiated thrombin generation above the baseline range (prior to anticoagulation) occurred within two minutes following a bolus administration of ANDEXXA and was maintained throughout the duration of the continuous infusion. The TF-initiated thrombin generation was elevated above placebo for at least 22 hours. The sustained elevation of thrombin generation over the baseline range and the sustained elevation over placebo were not observed in a contact-activated thrombin generation assay (an assay that is not affected by TF-TFPI interaction).
Laboratory assessment of coagulation does not necessarily correlate with or predict the hemostatic effectiveness of ANDEXXA.
12.2.1 Therapeutic Monitoring
Current commercial clinical anti-FXa-activity assays are unsuitable for measuring FXa activity following administration of ANDEXXA. Due to the reversible binding of ANDEXXA to the FXa inhibitor, the high sample dilution currently used in commercial clinical assays promotes dissociation of the inhibitor from ANDEXXA, resulting in detection of erroneously elevated anti-FXa activity levels, thereby causing a substantial underestimation of the reversal activity of ANDEXXA.
12.3. Pharmacokinetics
A summary of the pharmacokinetic (PK) properties of ANDEXXA in healthy subjects is shown in the table below (see Table 3).
Table 3. Summary of PK Parameters with High and Low Doses:
| Low Dose | High Dose | |
|---|---|---|
| n | 11 | 10 |
| AUC0-∞ (hr*µg/mL) | 200.5 (16.3) [153.4; 255.6] | 572.9 (16.0) [467.1; 783.9] |
| Cmax (µg/mL) | 76.6 (17.5) [61.1; 100.1] | 206.6 (18.8) [158.9; 280.5] |
| Clearance (L/hr) | 4.4 (16.3) [3.4; 5.7] | 3.1 (16.0) [2.3; 3.8] |
| T1/2 (hr) | 3.3 (15.0) [2.3; 4.0] | 2.7 (20.0) [1.9; 3.4] |
| Vss (L) | 4.4 (17.6) [3.3; 5.7] | 3.0 (23.3) [2.2; 5.0] |
From Table 14.2.1.2A of Clinical Study Report 16-512.
Data presented are geometric mean (Geometric Mean % Coefficient of Variation), [range].
Drug-Drug Interaction
The pharmacokinetics of ANDEXXA were not affected by apixaban (5 mg orally BID for six days) or rivaroxaban (20 mg orally once daily for six days).
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
No animal studies were performed to evaluate the effects of ANDEXXA on carcinogenesis, mutagenesis, or impairment of fertility.
14. Clinical Studies
The safety and efficacy of ANDEXXA were evaluated in two prospective, randomized, placebo-controlled studies, conducted in healthy volunteers (Study 1 ANNEXA-A; Study 2 ANNEXA-R). Both studies examined the percent change in anti-FXa activity, from baseline to nadir, for the low-dose and high-dose regimens of bolus followed by continuous infusion. Nadir is defined as the smallest value measured within five minutes after the end of the continuous infusion.
The safety and efficacy of ANDEXXA were evaluated in an ongoing, prospective, single-arm, open-label study (Study 3 ANNEXA-4) in subjects presenting with acute major bleeding and who have recently received an FXa inhibitor. This study examined the percent change in anti-FXa activity from baseline to the nadir between five minutes after the end of the bolus up until the end of the infusion and the rate of effective hemostasis within 12 hours after infusion, as rated by an independent endpoint adjudication committee.
Study 1 ANNEXA-A (NCT02207725) – apixaban reversal
In Study 1, healthy subjects (median age: 57 years; range: 50 to 73 years) received apixaban 5 mg twice daily for three and a half days to achieve steady-state. At three hours after the last apixaban dose (~Cmax), ANDEXXA or placebo was administered. Eight subjects received placebo, and 24 received ANDEXXA, administered as a 400 mg IV bolus followed by a 4 mg per minute continuous infusion for 120 minutes (total 480 mg).
Study 2 ANNEXA-R (NCT02220725) – rivaroxaban reversal
In Study 2, healthy subjects (median age: 57 years; range: 50 to 68 years) received rivaroxaban 20 mg once per day for four days to achieve steady-state. At four hours after the last rivaroxaban dose (~Cmax), ANDEXXA or placebo was administered. Thirteen subjects received placebo, and 26 received ANDEXXA, administered as an 800 mg IV bolus followed by an 8 mg per minute continuous infusion for 120 minutes (total 960 mg).
Reduction in Anti-FXa Activity
In Study 1 and Study 2, the percent change from baseline in anti-FXa activity at its nadir was statistically significant (p <0.0001) in favor of the ANDEXXA groups compared to placebo in both Studies 1 and 2. The results of Study 1 and Study 2 are provided below (see Table 4).
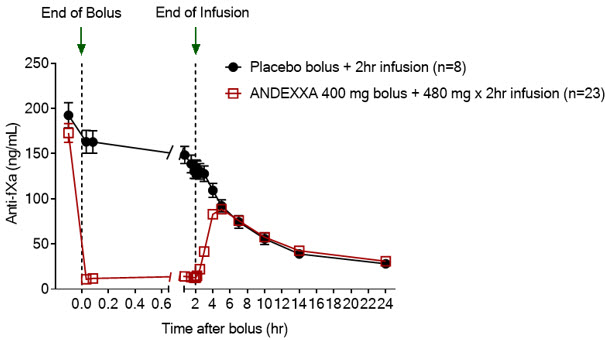
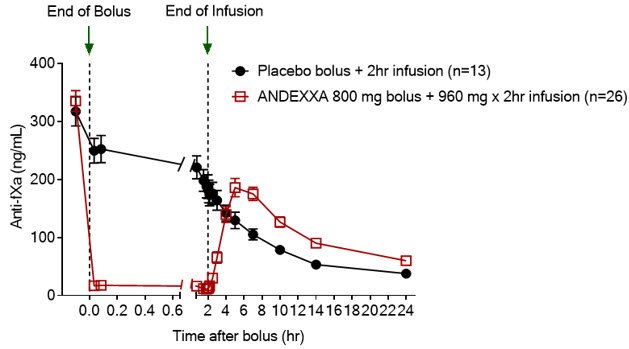
The time courses of anti-FXa activity before and after ANDEXXA administration are shown in Figure 1.
Table 4 – A. Change in Anti-FXa Activity/Study 1 (apixaban):
| Anti-FXa Activity | ANDEXXA n=23 | Placebo n=8 |
|---|---|---|
| Mean baseline ng/mL (± SD) | 173.0 (50.5) | 191.7 (34.4) |
| Mean ng/mL (± SD) change from baseline at the nadir* | -160.6 (49.3) | -63.2 (18.1) |
| Mean % (± SD) change from baseline at the nadir* | -92.3 (2.8) | -32.7 (5.6) |
| 95% confidence interval (CI)† | -59.5 (-64.1; -55.2) | not applicable |
| p-value | < 0.0001‡ | not applicable |
Table 4 – B. Change in Anti-FXa Activity/Study 2 (rivaroxaban):
| Anti-FXa Activity | ANDEXXA n=26 | Placebo n=13 |
|---|---|---|
| Mean baseline ng/mL (± SD) | 335.3 (91.0) | 317.2 (91.0) |
| Mean ng/mL (± SD) change from baseline at the nadir* | -324.5 (89.2) | -14.4 (58.8) |
| Mean % (± SD) change from baseline at the nadir* | -96.7 (1.8) | -44.6 (11.8) |
| 95% confidence interval (CI)† | -51.9 (-58.0; -47.0) | not applicable |
| p-value | < 0.0001‡ | not applicable |
SD = Standard deviation.
Note: Baseline is the last assessment obtained prior to the first dose of ANDEXXA or placebo.
* Nadir is the smallest value for anti-FXa activity at the 110-minute (ten minutes prior to the end of the infusion) time point, 2-minute time point before completion of the infusion, or the 5-minute time point after the completion of the infusion for each subject.
† The CI is for the Hodges-Lehman estimate of shift.
‡ p-value obtained from a 2-sided exact Wilcoxon rank-sum test.
Figure 1: Change in Anti-FXa Activity (ng/mL) in Subjects Anticoagulated with Apixaban (A – Study 1) and Rivaroxaban (B – Study 2)
Anti-FXa activity was measured prior to and after ANDEXXA or placebo administration. Dashed lines indicate the end of the bolus or infusion. A break in the x-axis is added to better visualize the immediate, short-term dynamics of anti-FXa activity following ANDEXXA treatment. The points on the graph represent the mean anti-FXa activity level; error bars illustrate standard error. There was a statistically significant difference (p < 0.05) in the percent change of anti-FXa activity normalized to pre-bolus between ANDEXXA and placebo until two hours after administration of infusion.
A. Apixaban – with ANDEXXA 400 mg IV bolus plus 4 mg/min infusion for 120 minutes.
B. Rivaroxaban – with ANDEXXA 800 mg IV bolus plus 8 mg/min infusion for 120 minutes.
Study 3 ANNEXA-4 (NCT02329327)
In an ongoing multinational, prospective, single-arm, open-label study, ANDEXXA was administered to 352 subjects taking FXa inhibitors who presented with acute major bleeding. The co-primary endpoints are: (a) percent change in anti-FXa activity from baseline to the nadir between five minutes after the end of the bolus up until the end of the infusion; and (b) rate of effective hemostasis within 12 hours after infusion, as rated by an independent endpoint adjudication committee.
Interim results of the study include data for 352 subjects dosed with ANDEXXA, of which 234 were efficacy-evaluable defined as subjects (1) on treatment with apixaban or rivaroxaban; (2) who had a baseline anti-FXa activity above 75 ng/mL; and (3) were adjudicated as meeting eligibility criteria for acute major bleeding [also see Adverse Reactions (6)].
For anti-FXa activity, the median decrease from baseline to nadir in anti-FXa activity for apixaban was -93% with a 95% CI of (-94%; -92%) and for rivaroxaban was -93% with a 95% CI of (-94%; -90%).
An improvement in hemostasis has not been established. ANDEXXA has not been shown to be effective for bleeding related to any FXa inhibitors other than apixaban or rivaroxaban.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.