Source: FDA, National Drug Code (US) Revision Year: 2020
ANDEXXA is indicated for patients treated with rivaroxaban or apixaban, when reversal of anticoagulation is needed due to life-threatening or uncontrolled bleeding.
This indication is approved under accelerated approval based on the change from baseline in anti-FXa activity in healthy volunteers [see Clinical Studies (14)]. An improvement in hemostasis has not been established. Continued approval for this indication may be contingent upon the results of studies that demonstrate an improvement in hemostasis in patients.
ANDEXXA has not been shown to be effective for, and is not indicated for, the treatment of bleeding related to any FXa inhibitors other than apixaban or rivaroxaban.
For intravenous (IV) use only.
There are two dosing regimens (see Table 1). The safety and efficacy of an additional dose have not been established.
Table 1. ANDEXXA Dosing Regimens:
| Dose* | Initial IV Bolus | Follow-On IV Infusion | Total Number of 200 mg Vials |
|---|---|---|---|
| Low Dose | 400 mg at a target rate of 30 mg/min | 4 mg/min for up to 120 minutes (480 mg) | 5 (2 vials bolus + 3 vials infusion) |
| High Dose | 800 mg at a target rate of 30 mg/min | 8 mg/min for up to 120 minutes (960 mg) | 9 (4 vials bolus + 5 vials infusion) |
The recommended dosing of ANDEXXA is based on the specific FXa inhibitor, dose of FXa inhibitor, and time since the patient’s last dose of FXa inhibitor (see Table 2).
Table 2. ANDEXXA Dose Based on Rivaroxaban or Apixaban Dose (Timing of Last Dose of FXa Inhibitor before ANDEXXA Initiation):
| FXa Inhibitor | FXa Inhibitor Last Dose | < 8 Hours or Unknown | ≥ 8 Hours |
|---|---|---|---|
| Rivaroxaban | ≤ 10 mg | Low Dose | Low Dose |
| > 10 mg or Unknown | High Dose | ||
| Apixaban | ≤ 5 mg | Low Dose | |
| > 5 mg or Unknown | High Dose |
| Determine total number of vials required (see Table 1). 200 mg vials: Reconstitute the 200 mg vial of ANDEXXA with 20 mL of Sterile Water for Injection (SWFI). Use a 20-mL (or larger) syringe and 20-gauge (or higher) needle. Slowly inject the SWFI, directing the solution onto the inside wall of the vial to minimize foaming. To reduce the total reconstitution time needed during preparation, reconstitute all required vials in succession. | 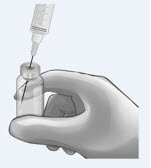 | |
| To ensure dissolution of the cake or powder, gently swirl each vial until complete dissolution of powder occurs (A). Do not shake (B); shaking could lead to foaming. Typical dissolution time for each vial is approximately three to five minutes. If dissolution is incomplete, discard the vial, and do not use the product. Upon reconstitution, the parenteral drug product should be inspected visually for particulate matter and discoloration prior to administration. | (A) | (B) |
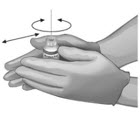 | 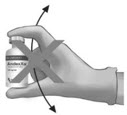 | |
| Use 60-mL (or larger) syringe with a 20-gauge (or higher) needle to withdraw the reconstituted ANDEXXA solution from each of the vials until the required dosing volume is achieved. Note the total volume withdrawn into the syringe. Transfer the ANDEXXA solution from the syringe into an empty polyolefin or polyvinyl chloride IV bag with a volume of 250 mL or less. | 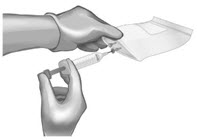 | |
Discard the syringe and needle.
Discard the vials, including any unused portion.
Patients treated with FXa inhibitor therapy have underlying disease states that predispose them to thromboembolic events. Reversing FXa inhibitor therapy exposes patients to the thrombotic risk of their underlying disease. To reduce the risk of thrombosis, resume anticoagulant therapy as soon as medically appropriate following treatment with ANDEXXA.
Unopened vials should be stored refrigerated at 2°C to 8°C (36°F to 46°F). DO NOT FREEZE.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.