CADUET Film-coated tablet Ref.[50597] Active ingredients: Amlodipine Atorvastatin Atorvastatin and Amlodipine
Source: Marketing Authorisation Holder Revision Year: 2023
Product description
CADUET (amlodipine and atorvastatin) tablets combine the calcium channel blocker amlodipine besylate with the HMG CoA-reductase inhibitor atorvastatin calcium.
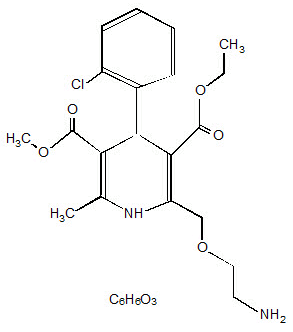
Amlodipine besylate is chemically described as 3-ethyl-5-methyl(±)-2-[(2-aminoethoxy)methyl]-4-(o-chlorophenyl)-1,4-dihydro-6-methyl-3,5-pyridinedicarboxylate, monobenzenesulphonate. Its empirical formula is C20H25ClN2O5∙C6H6O3S.
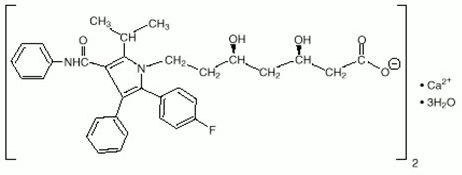
Atorvastatin calcium is chemically described as [R-(R*, R*)]-2-(4-fluorophenyl)-ß, δ-dihydroxy-5-(1-methylethyl)- 3-phenyl-4-[(phenylamino)carbonyl]-1H-pyrrole-1-heptanoic acid, calcium salt (2:1) trihydrate. Its empirical formula is (C33H34 FN2O5)2Ca∙3H2O.
The structural formulae for amlodipine besylate and atorvastatin calcium are shown below.
Amlodipine besylate:
Atorvastatin calcium:
CADUET contains amlodipine besylate, a white to off-white crystalline powder, and atorvastatin calcium, also a white to off-white crystalline powder. Amlodipine besylate has a molecular weight of 567.1 and atorvastatin calcium has a molecular weight of 1209.42. Amlodipine besylate is slightly soluble in water and sparingly soluble in ethanol. Atorvastatin calcium is insoluble in aqueous solutions of pH 4 and below. Atorvastatin calcium is very slightly soluble in distilled water, pH 7.4 phosphate buffer, and acetonitrile; slightly soluble in ethanol; and freely soluble in methanol.
CADUET is available as film-coated tablets containing:
- 5 mg amlodipine equivalent to 6.9 mg amlodipine besylate and 10 mg atorvastatin equivalent to 10.4 mg atorvastatin calcium.
- 5 mg amlodipine equivalent to 6.9 mg amlodipine besylate and 20 mg atorvastatin equivalent to 20.7 mg atorvastatin calcium.
- 5 mg amlodipine equivalent to 6.9 mg amlodipine besylate and 40 mg atorvastatin equivalent to 41.4 mg atorvastatin calcium.
- 5 mg amlodipine equivalent to 6.9 mg amlodipine besylate and 80 mg atorvastatin equivalent to 82.9 mg atorvastatin calcium.
- 10 mg amlodipine equivalent to 13.9 mg amlodipine besylate and 10 mg atorvastatin equivalent to 10.4 mg atorvastatin calcium.
- 10 mg amlodipine equivalent to 13.9 mg amlodipine besylate and 20 mg atorvastatin equivalent to 20.7 mg atorvastatin calcium.
- 10 mg amlodipine equivalent to 13.9 mg amlodipine besylate and 40 mg atorvastatin equivalent to 41.4 mg atorvastatin calcium.
- 10 mg amlodipine equivalent to 13.9 mg amlodipine besylate and 80 mg atorvastatin equivalent to 82.9 mg atorvastatin calcium.
Each film-coated tablet also contains calcium carbonate, croscarmellose sodium, microcrystalline cellulose, pregelatinized starch, polysorbate 80, hydroxypropyl cellulose, purified water, colloidal silicon dioxide (anhydrous), magnesium stearate, Opadry II White 85F28751 (polyvinyl alcohol, titanium dioxide, PEG 3000, and talc) or Opadry II Blue 85F10919 (polyvinyl alcohol, titanium dioxide, PEG 3000, talc, and FD&C blue #2).
| Dosage Forms and Strengths | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
CADUET tablets are formulated for oral administration in the following strength combinations: Table 1:
Combinations of atorvastatin with 5 mg amlodipine are film-coated white and combinations of atorvastatin with 10 mg amlodipine are film-coated blue. |
|||||||||||||||||||||
| How Supplied | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
CADUET tablets contain amlodipine besylate and atorvastatin calcium equivalent to amlodipine and atorvastatin in the dose strengths described below. CADUET tablets are differentiated by tablet color/size and are engraved with a unique number on one side. Combinations of atorvastatin with 5 mg amlodipine are oval and film-coated white and combinations of atorvastatin with 10 mg amlodipine are oval and are film-coated blue. CADUET tablets are supplied for oral administration in the following strengths and package configurations: Table 16. CADUET Packaging Configurations:
Distributed by: Pfizer Labs, Division of Pfizer Inc., New York, NY 10001 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drugs
| Drug | Countries | |
|---|---|---|
| CADUET | Austria, Australia, Canada, Spain, France, Hong Kong, Croatia, Japan, Lithuania, Malta, Mexico, Nigeria, Romania, Singapore, Tunisia, Turkey, United States, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.