CAMBIA Powder for oral solution Ref.[10765] Active ingredients: Diclofenac
Source: FDA, National Drug Code (US) Revision Year: 2019
Product description
CAMBIA (Diclofenac Potassium for Oral Solution) is a benzeneacetic acid derivative NSAID. CAMBIA is available as a buffered soluble powder, designed to be mixed with water prior to oral administration. CAMBIA is a white to off-white, buffered, flavored powder for oral solution packaged in individual unit dose packets [see How Supplied/Storage and Handling (16)].
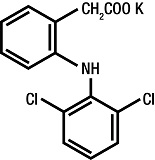
The chemical name for diclofenac potassium is 2-[(2,6-dichlorophenyl)amino] benzeneacetic acid monopotassium salt. The molecular weight of diclofenac potassium is 334.25. Its molecular formula is C14H10Cl2NKO2, and it has the following structural formula:
The inactive ingredients in CAMBIA include: aspartame (equivalent to 25 mg phenylalanine), flavoring agents (anise and mint), glycerol behenate, mannitol, potassium bicarbonate, and saccharin sodium.
| Dosage Forms and Strengths |
|---|
|
CAMBIA is available in individual packets each designed to deliver a 50 mg dose when mixed in water. |
| How Supplied |
|---|
|
CAMBIA 50 mg (Diclofenac Potassium for Oral Solution) is supplied as one or more sets of three perforated co-joined individual dose packets. Each individual packet is designed to deliver a dose of 50 mg diclofenac potassium when mixed in water. CAMBIA is a white to off-white, buffered, flavored powder for oral solution packaged in individual unit dose packets. Individual CAMBIA Packets – NDC 13913-011-01 Boxes of nine (9) CAMBIA Packets – NDC 13913-011-19 Manufactured by: MIPHARM S.p.A., Via Bernardo Quaranta, 12, 20141 Milan, Italy Manufactured for: Depomed, Inc., Newark, CA 94560, United States of America Manufactured and Distributed Under License from APR Applied Pharma Research SA, Balerna, Switzerland |
Drugs
| Drug | Countries | |
|---|---|---|
| CAMBIA | Canada, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.