CAMCEVI Prolonged-release suspension for injection Ref.[50038] Active ingredients: Leuprorelin
Source: European Medicines Agency (EU) Revision Year: 2023 Publisher: Accord Healthcare S.L.U., World Trade Center, Moll de Barcelona, s/n, Edifici Est 6ª planta, 08039, Barcelona, Spain
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Endocrine therapy, gonadotropin releasing hormone analogues
ATC code: L02AE02
Mechanism of action
Leuprorelin mesilate is a synthetic nonapeptide agonist of naturally occurring GnRH that, when given continuously, inhibits pituitary gonadotropin secretion and suppresses testicular steroidogenesis in males. This effect is reversible upon discontinuation of medicinal product therapy. However, the agonist possesses greater potency than the natural hormone and the time to recovery of testosterone levels may vary between patients.
Pharmacodynamic effects
Administration of leuprorelin results in an initial increase in circulating levels of luteinising hormone (LH) and follicle stimulating hormone (FSH), leading to a transient increase in levels of the gonadal steroids, testosterone and dihydrotestosterone in males. Continuous administration of leuprorelin results in decreased levels of LH and FSH. In males, testosterone is reduced to below castrate threshold (≤50 ng/dL).
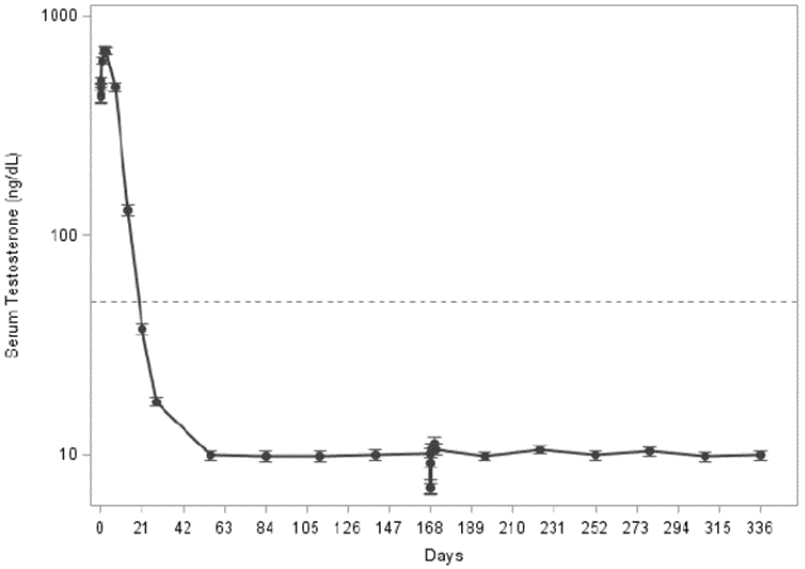
Following the first dose of leuprorelin, mean serum testosterone concentrations transiently increased, then fell to below castrate threshold levels (≤50 ng/dL) within 3-4 weeks, and were maintained below castrate threshold with 6-monthly administration of the medicinal product (Figure 1 below). Long-term studies on leuprorelin have shown that continuation of therapy maintains testosterone below the castrate level for up to seven years, and presumably indefinitely.
Tumour size was not measured directly during the clinical trial programme, but there was an indirect beneficial tumour response as shown by a 97% reduction in mean PSA for leuprorelin.
In a phase III randomised clinical study including 970 patients with locally advanced prostate cancer (mainly T2c-T4 with some T1c to T2b patients with pathological regional nodal disease) of whom 483 were assigned to short-term androgen suppression (6 months) in combination with radiation therapy and 487 to long-term therapy (3 years), a non-inferiority analysis compared the short-term to long- term concomitant and adjuvant hormonal treatment with GnRH agonist (triptorelin or goserelin). The 5-year overall mortality was 19.0% and 15.2%, in the short-term and long-term groups, respectively. The observed hazard ratio (HR) of 1.42 with an upper onesided 95.71% CI of 1.79 or two-sided 95.71% CI of 1.09; 1.85 (p=0.65 for non-inferiority), demonstrate that the combination of radiotherapy plus 6 months of androgen deprivation therapy provides inferior survival as compared with radiotherapy plus 3 years of androgen deprivation therapy. Overall survival at 5 years of long-term treatment and short-term treatment shows 84.8% and 81.0% survival, respectively. Overall quality of life using QLQ-C30 did not differ significantly between the two groups (p=0.37). Results are dominated by the population of patients with locally advanced tumours.
Evidence for the indication of high-risk localised prostate cancer is based on published studies of radiotherapy combined with GnRH analogues, including leuprorelin. Clinical data from five published studies were analysed (EORTC 22863, RTOG 85-31, RTOG 92-02, RTOG 8610, and D’Amico et al., JAMA, 2004), which all demonstrate a benefit for the combination of GnRH analogue with radiotherapy. Clear differentiation of the respective study populations for the indications locally advanced prostate cancer and high-risk localised prostate cancer was not possible in the published studies.
Clinical data have shown that radiotherapy followed by 3 years of androgen deprivation therapy is preferable to radiotherapy followed by 6 months of androgen deprivation therapy. The recommended duration of androgen deprivation therapy in medical guidelines for T3-T4 patients receiving radiotherapy is 2-3 years.
Clinical experience on efficacy with CAMCEVI
The multicentre, single-arm, open-label, 48-week phase 3 study of leuprorelin included 137 male patients with high-risk localised and locally advanced prostate cancer in need for androgen deprivation therapy. The efficacy of the medicinal product (two doses administered 24 weeks apart) was evaluated by the percentage of subjects with serum testosterone concentrations suppressed to castrate threshold levels, the effect on serum LH levels as measure for testosterone level control, and the effect on serum PSA levels. The percentage of patients with serum testosterone levels below the castrate threshold (≤50 ng/dL) by day 28 was 98.5% (135 out of 137 patients; intent-to-treat) and 99.2% (123 out of 124 subjects; per protocol), respectively (Figure 1).
Figure 1. Mean serum testosterone concentration over time with CAMCEVI (n=124; per protocol population):
Dotted line indicates the castrate level (50 ng/dL) of serum testosterone.
Mean serum LH levels were significantly reduced after the first injection, and this effect remained until the end of the study (decrease versus baseline by 98% [day 336]).
Tumour size was not directly measured in this study, but an indirect beneficial tumour response can be presumed for leuprorelin as shown by a significant reduction in mean PSA levels over time after injection of the medicinal product (mean of 70 ng/mL at baseline decreased to a mean minimum of 2.6 ng/mL [per-protocol population] at Day 168.
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with the reference medicinal product containing leuprorelin in all subsets of the paediatric population in prostate carcinoma (see section 4.2 for information on paediatric use).
5.2. Pharmacokinetic properties
Absorption
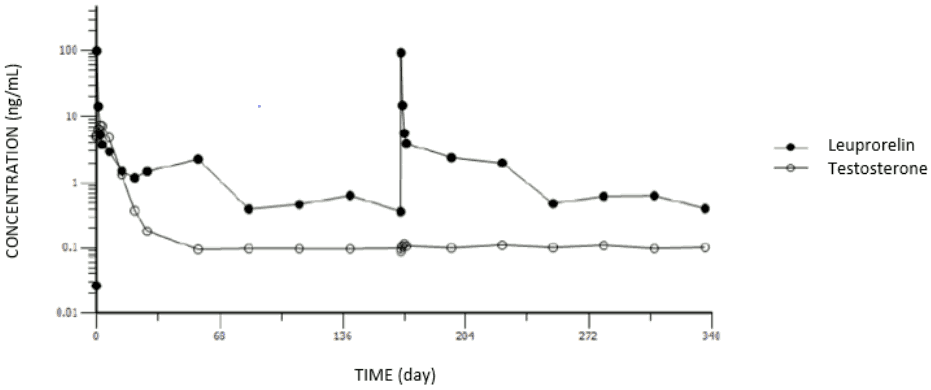
Following the first and the second doses of leuprorelin, an initial rapid increase of serum leuprorelin concentration was observed, followed by a rapid decline over the first 3 days postdose: after an initial “burst” phase characterised by mean serum leuprorelin concentrations of 99.7 and 93.7 ng/mL after approximately 3.7 and 3.8 hours post dosing, respectively, mean serum leuprorelin levels maintained relatively constant over each 24-week dosing interval, with leuprorelin being continuously released by the third day after dosing with steady serum concentrations (“plateau” phase) through the 24-week (approximately 6-month) dosing interval (mean concentration: 0.37 to 2.97 ng/mL). There is no indication of significant accumulation with repeated leuprorelin dosing at 24-week intervals.
The initial acute increase of leuprorelin concentrations after CAMCEVI are followed by a rapid decline to a steady state levels.
The pharmacokinetics/pharmacodynamics (as per serum testosterone level) profiles of leuprorelin versus serum testosterone level observed after initial injection of CAMCEVI (first dose) and at 24 weeks (second dose) is shown in Figure 2 (study FP01C-13-001; Part II).
Figure 2. Pharmacokinetic/pharmacodynamic response to CAMCEVI:
Distribution
The mean steady-state volume of distribution of leuprorelin following intravenous bolus administration to healthy male volunteers was 27 litres. In-vitro binding to human plasma proteins ranged from 43% to 49%.
Metabolism
No metabolism study was conducted with leuprorelin.
Elimination
In healthy male volunteers, a 1 mg bolus of leuprorelin administered intravenously revealed that the mean systemic clearance was 8.34 L/h, with a terminal elimination half-life of approximately 3 hours based on a two-compartment model.
No excretion studies have been conducted with leuprorelin.
5.3. Preclinical safety data
Preclinical studies with leuprorelin revealed in both sexes effects on the reproductive system, which were expected from the known pharmacological properties. These effects were shown to be reversible after discontinuation of the treatment and an appropriate period of regeneration. Leuprorelin did not show teratogenicity. Embryotoxicity/lethality was observed in rabbits, in line with the pharmacological effects of leuprorelin on the reproductive system.
Consistent with the GnRH agonistic effects of leuprorelin hyperplasia and adenoma were observed in the anterior pituitary of rats.
Carcinogenicity studies were performed in rats and mice over 24 months. In rats, a dose-related increase in pituitary apoplexy was observed after subcutaneous administration at doses of 0.6 to 4 mg/kg/day. No such effect was observed in mice.
Leuprorelin was not mutagenic in a set of in-vitro and in-vivo assays.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.