CAMCEVI Prolonged-release suspension for injection Ref.[50038] Active ingredients: Leuprorelin
Source: European Medicines Agency (EU) Revision Year: 2023 Publisher: Accord Healthcare S.L.U., World Trade Center, Moll de Barcelona, s/n, Edifici Est 6ª planta, 08039, Barcelona, Spain
4.1. Therapeutic indications
CAMCEVI is indicated for the treatment of hormone dependent advanced prostate cancer and for the treatment of high-risk localised and locally advanced hormone dependent prostate cancer in combination with radiotherapy.
4.2. Posology and method of administration
Posology
Adult prostate cancer patients
CAMCEVI should be administered under the direction of a healthcare professional having available the appropriate expertise for monitoring the response to treatment.
CAMCEVI 42 mg is administered as a single subcutaneous injection every six months. The injected suspension forms a solid medicinal product delivery depot and provides continuous release of leuprorelin over a six-month period.
As a rule, therapy of advanced prostate cancer with leuprorelin entails long-term treatment and therapy should not be discontinued when remission or improvement occurs.
Leuprorelin may be used as neoadjuvant or adjuvant therapy in combination with radiotherapy in high-risk localised and locally advanced prostate cancer.
Response to leuprorelin should be monitored by clinical parameters and by measuring prostate specific antigen (PSA) serum levels. Clinical studies have shown that testosterone levels increased during the first 3 days of treatment in the majority of non-orchiectomised patients and then decreased to below medical castration levels within 3 to 4 weeks. Once attained, castrate levels were maintained as long as leuprorelin therapy continued (<1% testosterone breakthroughs). In case the patient’s response appears to be sub-optimal, it should be confirmed that serum testosterone levels have reached or are remaining at castrate levels.
In patients with metastatic castration resistant prostate cancer not surgically castrated receiving a gonadotropin-releasing hormone (GnRH) agonist, such as leuprorelin, and eligible for treatment with androgen biosynthesis inhibitors or androgen receptor inhibitors, treatment with a GnRH agonist may be continued.
Special populations
Renal/hepatic impairment
No clinical studies were performed in patients having either renal or hepatic impairment.
Paediatric population
The safety and efficacy of leuprorelin in children aged 0 to 18 years have not been established (see also section 4.3). No data are available.
Method of administration
CAMCEVI should be prepared and administered subcutaneously only by healthcare professionals who are familiar with these procedures. For instructions on preparation and administration of the medicinal product, see section 6.6.
Intra-arterial or intravenous injection, respectively, has to be strictly avoided.
As with other medicinal products administered by subcutaneous injection, the injection site should be varied periodically.
4.9. Overdose
Leuprorelin does not have the potential for abuse, and deliberate overdose is unlikely. There are no reports of abuse or overdose having occurred in clinical practice with leuprorelin, but in the event that excessive exposure becomes a reality, observation and symptomatic supportive treatment are recommended.
6.3. Shelf life
2 years.
6.4. Special precautions for storage
Store in a refrigerator (2°C–8°C).
Store in the original package in order to protect from light.
6.5. Nature and contents of container
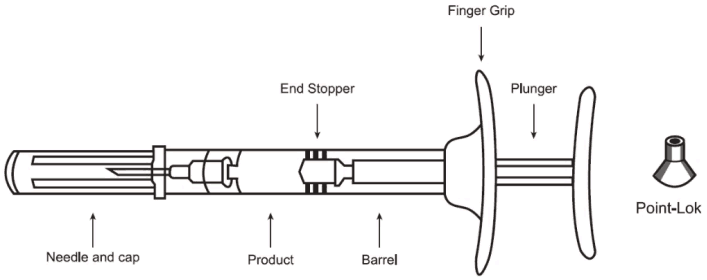
One pack contains: 1 pre-filled syringe (cyclic olefin copolymer, closed with bromobutyl elastomeric grey tip cap, plunger and finger grip), 1 needle (18 gauge, 5/8 inch) and 1 Point-Lok needle protection device.
6.6. Special precautions for disposal and other handling
Follow the instructions as directed to ensure proper preparation of CAMCEVI prior to administration.
Important: Prior to use allow CAMCEVI to reach room temperature (15 °C to 25 °C). The use of gloves is recommended during administration.
CAMCEVI contains:
- One blister with:
- One sterile pre-filled syringe;
- One sterile needle.
- One Point-Lok needle protection device (non-sterile).
Assembled pre-filled syringe, including Point-Lok:
Step 1 – Prepare the medicinal product:
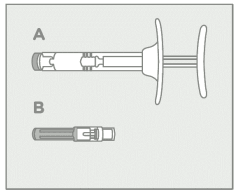 | Allow to reach room temperature and inspect contents • Remove CAMCEVI from refrigerator. • Prior to use allow CAMCEVI to reach room temperature (15°C to 25°C). This takes approximately 15 to 20 minutes. • On a flat, clean and dry surface open carton and remove pre-filled CAMCEVI syringe (A) and needle protected with a cap (B) from the blister container. Examine all contents of the package. Do not use if any component is damaged. • Place the Point-Lok needle protection device, supplied within the CAMCEVI, on a secured flat surface. • Check the expiry date on the syringe. Do not use if the expiry date has passed. • Visually inspect the medicine prior to use. The pre-filled syringe should contain off- white to pale yellow viscous and opalescent suspension. Do not use if foreign particles are noticed inside the syringe barrel. |
Step 2 – Syringe assembly:
Attach the needle 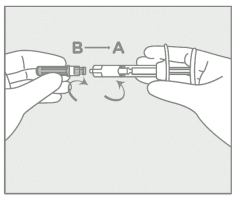 | • Remove the grey cap from the syringe (A). • Twist the clear cap off the bottom of the needle (B). • Attach the needle (B) to the end of the syringe (A) by pushing and turning until firmly connected. Do not over twist the needle and strip the threading to avoid possible breakage and drug leakage. Discard pre-filled CAMCEVI syringe if over-twist causes syringe breakage. |
Step 3 – Administration procedure:
Prepare the injection site 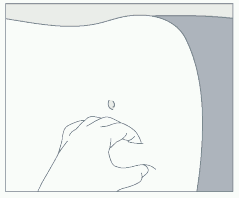 Administer treatment 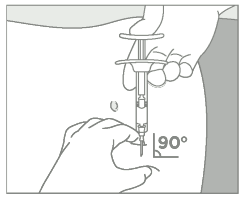 | • Choose an injection site on the upper- or mid-abdominal area with sufficient soft or loose subcutaneous tissue that has not recently been used. The injection site should be varied periodically. • Clean the injection site with an alcohol swab. Do NOT inject in areas with brawny or fibrous subcutaneous tissue or locations that can be rubbed or compressed (i.e., with a belt or clothing waistband). • Pull the blue cover off the needle (B). Grab and bunch the skin around the injection site with one hand. Insert the needle at a 90° angle, then release the bunched skin. • Inject the full contents of the syringe with a slow and steady push, then withdraw the needle at the same 90° angle used for insertion. Intra-arterial or intravenous injection have to be strictly avoided. |
Step 4 – Discard needle and pre-filled syringe:
Needle Protection 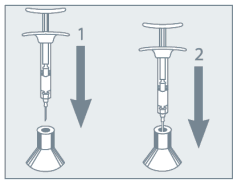 | • Do not remove the needle from the syringe. Use the enclosed Point-Lok device to prevent needle sticks. • Immediately after use of the needle, gently insert the exposed needle into the Point-Lok device opening at top of the device. • Push needle into the top opening until it is firmly inserted into the Point-Lok device. This action will seal the needle tip and lock the needle firmly into the device. • After use, place the used syringe with needle protected in a suitable sharps container. Any unused medicinal product or waste material should be disposed of in accordance with local requirements. |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.